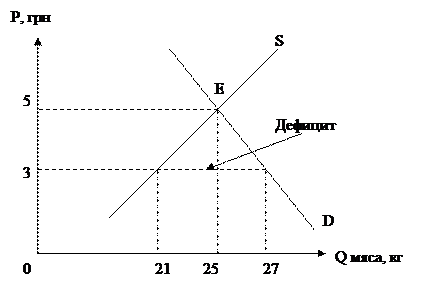
Solution - раствор
Dmitriy Ivanovich Mendeleev was a Russian chemist. He is credited as being the primary creator of the first version of the periodic table of elements. Mendeleev was born in Tobolsk, Siberia. Mendeleev was the 13th surviving child of 17 total, but the exact number differs among sources. At the age of 13 Mendeleev attended the Gymnasium in Tobolsk. In 1849, the now poor Mendeleev family relocated to St. Petersburg, where he entered the Main Pedagogical Institute in 1850. After he graduated, an illness that was diagnosed as tuberculosis caused him to move to the Crimean Peninsula on the northern coast of the Black Sea in 1855. While there he became chief science master of the local gymnasium. He returned with fully restored health to St. Petersburg in 1857. Between 1859 and 1861, he worked on the capillarity of liquids and the workings of the spectroscope in Heidelberg. Mendeleev became Professor of Chemistry at the Saint Petersburg Technological Institute and the University of St. Petersburg in 1863, achieved tenure in 1867, and by 1871 had transformed St. Petersburg into an internationally recognized center for chemistry research. In 1865 he became Doctor of Science for his dissertation "On the Combinations of Water with Alcohol". In 1893, he was appointed Director of the Bureau of Weights and Measures. As a result of his work, in 1894 new standards for vodka were introduced into Russian law and all vodka had to be produced at 40% alcohol by volume. Mendeleev also investigated the composition of oil fields, and helped to found the first oil refinery in Russia. After becoming a teacher, he wrote the definitive two-volume textbook at that time: Principles of Chemistry. On March 6, 1869, Mendeleev made a formal presentation to the Russian Chemical Society, which described elements according to both weight and valence. The elements, if arranged according to their atomic mass, exhibit an apparent periodicity of properties. Elements which are similar as regards to their chemical properties have atomic weights which are either of nearly the same value or which increase regularly. The arrangement of the elements in groups of elements in the order of their atomic weights, corresponds to their so-called valencies, as well as, to some extent, to their distinctive chemical properties. The elements which are the most widely diffused have small atomic weights. The magnitude of the atomic weight determines the character of the element, just as the magnitude of the molecule determines the character of a compound body. The atomic weight of an element may sometimes be amended by a knowledge of those of its contiguous elements. Certain characteristic properties of elements can be foretold from their atomic weights. The Russian chemist and science historian L.A. Tchugayev has characterized him as "a chemist of genius, first-class physicist, a fruitful researcher in the fields of hydrodynamics, meteorology, geology, certain branches of chemical technology (explosives, petroleum, and fuels, for example) and other disciplines adjacent to chemistry and physics, a thorough expert of chemical industry and industry in general, and an original thinker in the field of economy." Mendeleev was one of the founders, in 1869, of the Russian Chemical Society. He worked on the theory and practice of protectionist trade and on agriculture. Mendeleev devoted much study, and made important contributions to, the determination of the nature of such indefinite compounds as solutions. Mendeleev is given credit for the introduction of the metric system to the Russian Empire. He invented pyrocollodion, a kind of smokeless powder based on nitrocellulose. Mendeleev studied petroleum origin and concluded that hydrocarbons are abiogenic and form deep within the earth. Mendeleev died in 1907 in St. Petersburg, Russia from influenza. The Mendeleev crater on the Moon, as well as element number 101, the radioactive mendelevium, are named after him. Exercise 19. Answer the questions: 1. Where was Mendeleev born? 2. What institute did Mendeleev enter in St. Petersburg? 3. What made the Russian chemist move to the Crimean peninsula? 4. Can you name the theme of Mendeleev’s dissertation? 5. When did Mendeleev make a presentation to the Russian chemical society? 6. Speak about the periodical table of elements. 7. Enumerate all the achievements of the great chemist in different scientific fields. 8. What is pyrocollodion? Exercise 20. Give the English equivalents to the following expressions: Химик, периодическая система элементов, точное число, болезнь, полуостров, местный, химическое исследование, новые стандарты, нефтяные месторождения, валентность, атомная масса, периодичность свойств, химические свойства, регулярно увеличиваться, так называемые, определять, характер элемента, историк, гениальный химик, плодотворный исследователь, взрывчатые вещества, в общем, основатель, вклад, империя, кратер, посвятить, метрическая система. Exercise 21. Give the Russian equivalents to the following expressions: the primary creator, the first version, surviving child, chief science master, to achieve tenure, doctor of Science, to investigate, oil refinery, similar, the same value, the arrangement of the elements, compound body, certain branches, thorough expert, thinker, indefinite compounds, smokeless powder, petroleum origin, within the earth. Exercise 22. Are the following sentences true or false? If they are false, give the right answers. 1. Mendeleev was born in St. Petersburg. 2. He was the 10th child in the family. 3. The illness made Mendeleev move to the northern coast of the Black sea. 4. One of the satellites is named after Mendeleev. 5. The Russian chemical Society was founded in 1869. 6. Pyrocolloidon, invented by Mendeleev, is a kind of solutions. Exercise 23. Insert the missing words: 1. Mendeleev is…of the periodic table of elements. 2. The periodic table of elements is based on… and… 3. Element number 101, called.., is named after Mendeleev.
|