Molecular Biochemistry I
http://www.rpi.edu/dept/bcbp/molbiochem/MBWeb/mb1/part2/redox.htm#respchain
Contents of this page:
Electron transfer reactions
Electron carriers
Respiratory chain
Electron Transfer is discussed on p. 555-556, 571-574 and 802-820 of Biochemistry, 3rd Edition, by Voet & Voet.
Aox + Bred «Ared + Box
- Aox is the oxidized form of A (the oxidant in the reaction shown)
- Bred is the reduced form of B (the reductant).
For such an electron transfer, one may consider two half-cell reactions:
- Aox + n e- «Ared......e.g., Fe+++ + e- «Fe++
- Box + n e- «Bred
For each half reaction:
E = E°' - RT/nF (ln [reduced]/[oxidized])
e.g., for the first half reaction:
E = E°' - RT/nF (ln [Ared]/[Aox])
E = voltage, R = Gas Constant, F = Faraday Constant, n = number of e- transferred.
When [Ared] = [Aox], .. E = E°'.
E°' is the mid-point potential, or standard redox potential. It is the potential at which [oxidant] = [reductant] for the half reaction.
For an electron transfer:
DE°' = E°'(oxidant) - E°'(reductant) = E°'(e- acceptor) - E°'(e- donor)
DGo' = - nFDEo'
An electron transfer is spontaneous (negative DG) if E°' (mid-point potential) of the e- donor is more negative than E°' of the e- acceptor, i.e., when there is a positive DE°'.
Consider, for example, transfer of 2 electrons from NADH to oxygen:
| a. ЅO2 + 2H+ + 2 e- ® H2O
| E°' = + 0.815 V
|
| b. NAD+ + 2H+ + 2 e- ® NADH + H+
| E°' = - 0.315 V
|
| Subtracting reaction b from reaction a:
|
|
| ЅO2 + NADH + H+ ® H2O + NAD+
| DE°' = + 1.13 V
|
DGo' = - nFDEo' = - 2(96485 Joules/Volt · mol)(1.13 V) = - 218 kJ/mol
Electron carriers:
NAD+/NADH and FAD/FADH2 were introduced in the class on bioenergetics.
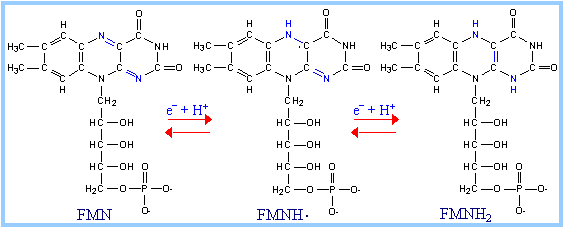
FMN (F lavin M ono N ucleotide) is a prosthetic group of some flavoproteins. It is similar in structure to FAD (Flavin Adenine Dinucleotide), but lacking the adenine nucleotide.
FMN (like FAD) can accept 2 e- + 2 H+ to yield FMNH2. When bound at the active site of some enzymes, FMN can accept 1 e-, converting it to the half-reduced semiquinone radical. The semiquinone can accept a second e- to yield FMNH2.
| Role of FMN: Since it can accept/donate either 1 or 2 e-, FMN has an important role in mediating electron transfer between carriers that transfer 2 e- (e.g., NADH) and carriers that can only accept 1 e- (e.g., Fe+++). See discussion of complex I below.
| 
|
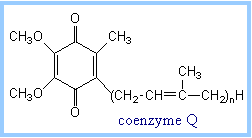
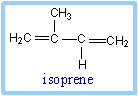
| Coenzyme Q (also called CoQ, Q or ubiquinone) is very hydrophobic. It dissolves in the hydrocarbon core of a membrane. The structure of CoQ includes a long isoprenoid tail, with multiple units having a carbon skeleton comparable to that of the compound isoprene. In human cells, most often the number of isoprene units (n) is 10.
The isoprenoid tail of Q10 is longer than the width of a lipid bilayer. The isoprenoid moiety of CoQ may be folded to yield a more compact structure, and is postulated to reside in the central hydrophobic domain of a membrane, between the two lipid monolayers.
|  
|
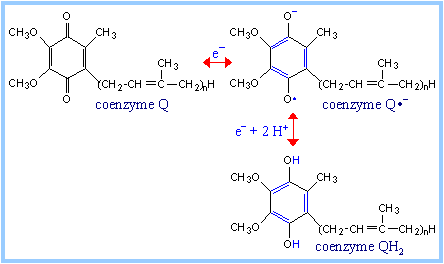
| The quinone ring of coenzyme Q can be reduced to a quinol in a 2e- reaction:
Q + 2e- + 2H+ «QH2
When bound to special sites in respiratory chain complexes, CoQ can accept a single electron to form a semiquinone radical (Q·-). Thus CoQ, like FMN, can mediate between one-electron and two-electron donors/acceptors.
Coenzyme Q functions as a mobile electron carrier within the mitochondrial inner membrane. Its role intransmembraneH+ transport coupled to electron transfer (the Q Cycle) will be discussed in the section on oxidative phosphorylation.
| 
|
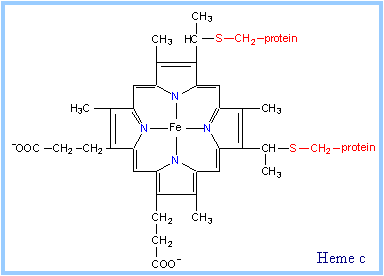
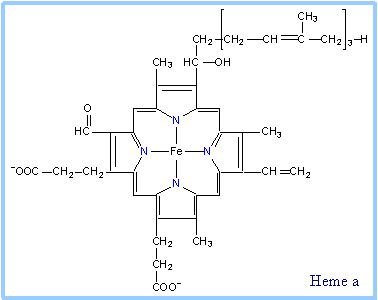
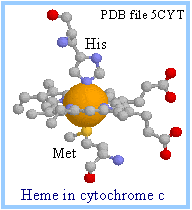
| Heme is a prosthetic group of cytochromes. Heme contains an iron atom embedded in a porphyrin ring system, shown at right & below. The Fe is bonded to 4 N atoms of the porphyrin ring.
Hemes in the three classes of cytochrome (a, b, c) differ slightly in substituents on the porphyrin ring system (see p. 813). A common feature is two propionate side-chains.
Only heme c is covalently linked to the protein via thioether bonds to cysteine residues, as shown at right.
| 
|
| Heme a is unique in having a long farnesyl side-chain that includes three isoprenoid units.
Synthesis of heme is discussed separately.
| 
|
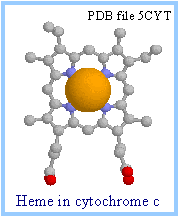
| In the RasMol display of heme c at right, the porphyrin ring is displayed as ball & stick, while the iron is shown as spacefill. Iron is colored gold and nitrogen blue.
The heme iron atom can undergo a 1 e- transition between ferric and ferrous states: Fe+++ + e-«Fe++
| 
|
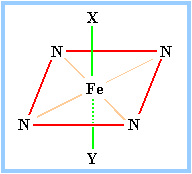
| The porphyrin ring structure is planar. The iron atom of heme is usually bonded to two axial ligands, above and below the heme plane (X & Y in the diagram at right), in addition to the 4 N of the porphyrin ring system. Axial ligands may be sulfur or nitrogen atoms of amino acid side-chains of the protein.
| 
|
Axial ligands in cytochrome c are a methionine S (yellow) and a histidine N (blue), as shown at right. A heme that binds O2 may have an open (empty) axial ligand position.
Cytochromes are proteins with heme prosthetic groups. They absorb light at characteristic wavelengths. Changes in light absorbance upon oxidation or reduction of the heme iron provide a basis for monitoring the redox state of the heme.
- Some cytochromes are part of large integral membranecomplexes, each consisting of several polypeptides and including multiple electron carriers. Individual heme prosthetic groups may be separately designated as cytochromes, even if associated with the same protein. For example, hemes a and a3 that are part of the respiratory chain complex IV are often referred to as cytochromes a and a3.
- Cytochrome c is instead a small, water-soluble protein, with a single heme group.
| 
|
| Positively charged lysine residues surround the heme crevice on the surface of cytochrome c. These may interact with anionic residues on membrane complexes to which cytochrome c binds, when it is receiving or donating an electron (diagram below).
In the image at right, Lys residues are colored magenta; all atoms are displayed as spacefill except for the porphyrin ring of heme which is in ball and stick display.
| 
|

| Explore the structure of cytochrome c at right.
|
 Cytochrome c Cytochrome c
|
| Iron-sulfur centers (Fe-S) are prosthetic groups containing 2, 3, 4, or 8 iron atoms, complexed to a combination of elemental and cysteine sulfur atoms.
4-Fecenters have a tetrahedral structure, with Fe and S atoms alternating as vertices of a cube, as depicted at right. See also diagrams p. 808 & 809.
The cysteine residues provide sulfur ligands to the iron, while also holding these prosthetic groups in place within the protein.
Electron transfer proteins may contain multiple iron-sulfur centers.
| 
|
| Iron-sulfur centers transfer only one electron even if they contain two or more iron atoms, because of the close proximity of the iron atoms.
For example a 4-Fe center might cycle between the redox states: Fe+++3, Fe++1 (oxidized) + 1 e-«Fe+++2, Fe++2(reduced)
| 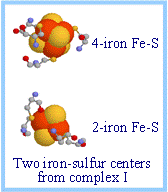 Iron-sulfur centers in spacefill display; cysteines in ball & stick display. Iron is red-orange; sulfur is yellow. Data from PDB file 2FUG. Iron-sulfur centers in spacefill display; cysteines in ball & stick display. Iron is red-orange; sulfur is yellow. Data from PDB file 2FUG.
|
| Respiratory chain
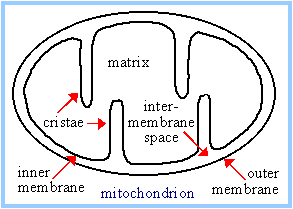
Most constituents of the respiratory chain are embedded in the inner mitochondrial membrane (or in the cytoplasmic membrane of aerobic bacteria). The inner mitochondrial membrane has infoldings called cristae that increase the membrane area.
| 
|
| Electrons are transferred from NADH to O2 via multi-subunit inner membrane complexes I, III, & IV, plus coenzyme Q and cytochrome c. Within each complex, electrons pass sequentially through a series of electron carriers.
Coenzyme Q is located within the lipid core of the inner membrane. There are also binding sites for coenzyme Q within protein complexes with which it interacts.
Cytochrome c resides in the intermembrane space (within the lumen of the cristae). It alternately binds to Complex III or Complex IV during electron transfer.
| 
|
Individual respiratory chain complexes have been isolated and their composition determined. There is also evidence for the existence of stable supramolecular aggregates containing multiple complexes. E.g., complex I, which transfers electrons to coenzyme Q, may associate with complex III, which reoxidizes the reduced coenzyme Q, to provide a pathway for direct transfer of coenzyme Q between them.
The composition of each of the respiratory chain complexes is shown below and in Table 22-1 p. 806.
| Complex
| Name
| No. of Proteins
| Prosthetic Groups
|
| Complex I
| NADH Dehydrogenase
|
| FMN, 9 Fe-S centers
|
| Complex II
| Succinate-CoQ Reductase
|
| FAD, cyt b560, 3 Fe-S centers
|
| Complex III
| CoQ-cyt c Reductase
|
| cyt bH, cyt bL, cyt c1, Fe-SRieske
|
| Complex IV
| Cytochrome Oxidase
|
| cyt a, cyt a3, CuA, CuB
|
The approximate mid-point potentials of constituent electron carriers is represented in the diagram on p. 803 and in table 22-1 on p. 806. The mid-point potentials are consistent with the electron transfers shown above being spontaneous.
Respiratory chain inhibitors include the following:
Rotenone (a common rat poison) blocks electron transfer in complex I.
Antimycin A blocks electron transfer in complex III.
Cyanide and carbon monoxide inhibit complex IV.
Inhibition at any of these sites will block electron transfer from NADH to oxygen.
Complex I catalyzes oxidation of NADH, with reduction of coenzyme Q:
NADH + H+ + Q ® NAD+ + QH2
Transmembrane H+ flux associated with this reaction is discussed in the section on oxidative phosphorylation.
| An atomic-level structure is not yet available for the entirecomplex I, which in mammals includes at least 46 proteins, along with prosthetic groups FMN and several iron-sulfur centers.
Complex I is L-shaped. For a low-resolution structure determined by electron microscopy see diagram in the textbook p. 810.
The peripheral domain of the complex, containing the FMN that accepts 2 electrons from NADH, protrudes into the mitochondrial matrix. Iron-sulfur centers are also located in the hydrophilic peripheral domain, where they form a pathway for electron transfer from FMN to coenzyme Q. A binding site for coenzyme Q is thought be close to the interface between peripheral and intra-membrane domains.
| 
|
The initial electron transfers are:
NADH + H+ + FMN ® NAD+ + FMNH2
FMNH2 + (Fe-S)ox® FMNH· + (Fe-S)red + H+
After Fe-S is reoxidized by transfer of the electron to the next iron-sulfur center in the pathway:
FMNH· + (Fe-S)ox® FMN + (Fe-S)red + H+
Electrons pass through a series of iron-sulfur centers, and are eventually transferred to coenzyme Q. Coenzyme Q accepts 2 e- and picks up 2 H+ to yield the fully reduced QH2.
An X-ray structure has been determined for the hydrophilic peripheral domain of a bacterial complex I. This bacterial complex I contains fewer proteins than the mammalian complex I, but includes the central subunits found in all prokaryotic and eukaryotic versions of complex I.
The prosthetic groups are found to be all in the peripheral domain, that in mammalian complex I would protrude into the mitochondrial matrix.
Iron-sulfur centers are arranged as the a wire, providing a pathway for electron transfer from FMN through the protein. The last iron-sulfur center in the chain, designated N2, passes electrons one at a time to the mobile lipid redox carrier coenzyme Q. A proposed binding site for coenzyme Q is close to N2 at the interface of peripheral & membrane domains.
P. L. Dutton and coworkers have called attention to the relevance of conserved distances between redox carriers within respiratory chain complexes with regard to the energy barrier at each step for electron tunneling through the protein. They have modeled electron transfers through the respiratory chain complexes, and provide an animation of the time course of electron transfer through Complex I. (For details see the article by Moser et al., also included in the reference list.)
For more diagrams and additional information see:
- A review by U. Brandt (requires Annual Reviews subscription.)
- The Complex I Home Page
| 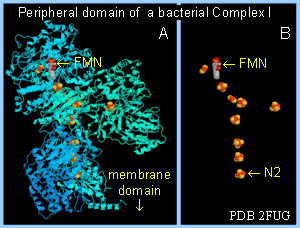 Peripheral domain of complex I from T. thermophilus. A. Protein in cartoon display; FMN & FeS centers spacefill. B. Same but with protein hidden. Structure published by Sazanov & Hinchcliffe in 2006. Peripheral domain of complex I from T. thermophilus. A. Protein in cartoon display; FMN & FeS centers spacefill. B. Same but with protein hidden. Structure published by Sazanov & Hinchcliffe in 2006.
|
| Succinate Dehydrogenase of the Krebs Cycle is also called complex II or Succinate-CoQ Reductase.
FAD is the initial electron acceptor. FAD is reduced to FADH2 during oxidation of succinate to fumarate. FADH2 is then reoxidized by transfer of electrons through a series of three iron-sulfur centers to Coenzyme Q, yielding QH2. The QH2 product may then be reoxidized via complex III, providing a pathway for transfer of electrons from succinate into the respiratory chain.
| 
|
| X-ray crystallographic analysis of E. coli complex II indicates a linear arrangement of electron carriers within complex II, consistent with the predicted sequence of electron transfers: FAD ® FeScenter 1 ® FeScenter 2 ® FeScenter 3 ® CoQ
In the crystal structure at right, oxaloacetate (colored black) is bound at the active site in place of succinate. See also diagram p. 811.
| 
|
Complex III accepts electrons from coenzyme QH2 that is generated by electron transfer in complexes I and II. The structure and roles of complex III are discussed in the section on oxidative phosphorylation. Cytochrome c1, a prosthetic group within complex III, reduces cytochrome c, which is the electron donor to complex IV.
| Cytochrome oxidase (complex IV) carries out the following irreversible reaction: O2 + 4 H+ + 4 e- ® 2 H2O
The four electrons are transferred into the complex one at a time from cytochrome c.
Intramembrane domains of cytochrome oxidase (complex IV) consist mainly of transmembrane a-helices.
| 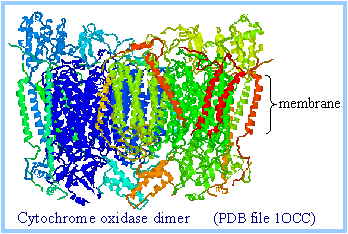
|
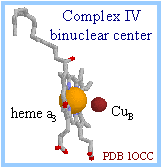
| Metal centers of cytochrome oxidase (complex IV) include heme a, heme a3, CuA (consisting of 2 adjacent Cu atoms) and CuB. O2 reacts at a binuclear center, consisting of heme a3 and CuB. In the diagram at right, the iron atom of heme a3 in gold, and copper atom in dark red, are displayed as spacefill, with the heme displayed as sticks.
| 
|
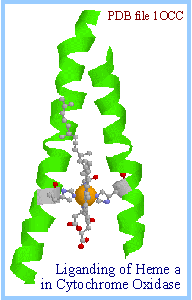
| Metal center ligands:
Axial ligands of the hemes in complex IV are histidine N atoms. Note at right how heme a is held in place within the complex, between 2 transmembrane a-helices, by its axial histidine ligands. Heme a3, which sits adjacent to CuB, has only one such axial ligand (diagram below right).
Ligands of Cu atoms consist of histidine N, and in the case CuA also cysteine S, a methionine S, and a glutamate backbone O.
Electrons enter complex IV one at a time by transfer from cytochrome c to CuA. They then pass via heme ato the binuclear center consisting of heme a3 and CuB, where the chemical reaction takes place.
Order of e- transfers: cyt c → CuA → heme a → heme a3/CuB
| 
|
| O2binds at the open axial ligand position of heme a3, adjacent to CuB. A possible reaction sequence is depicted on p. 819. Details of the reaction are still debated. A tyrosine-histidine complex adjacent to the binuclear center is postulated to have a role in O-O bond splitting. Diagram p. 818.
Proton pumping linked to electron transfer in complex IV will be discussed separately.
The open axial ligand position of the iron atom in heme a3 makes it susceptible to binding each of the following inhibitors: CN-, CO, and the radical signal molecule ·NO (nitric oxide).
·NO may regulate cellular respiration through its inhibitory effect, and can induce a condition comparable to hypoxia.
| 
|
| Explore at right the structure of cytochrome oxidase (complex IV).
|












 Cytochrome c
Cytochrome c

 Iron-sulfur centers in spacefill display; cysteines in ball & stick display. Iron is red-orange; sulfur is yellow. Data from PDB file 2FUG.
Iron-sulfur centers in spacefill display; cysteines in ball & stick display. Iron is red-orange; sulfur is yellow. Data from PDB file 2FUG.



 Peripheral domain of complex I from T. thermophilus. A. Protein in cartoon display; FMN & FeS centers spacefill. B. Same but with protein hidden. Structure published by Sazanov & Hinchcliffe in 2006.
Peripheral domain of complex I from T. thermophilus. A. Protein in cartoon display; FMN & FeS centers spacefill. B. Same but with protein hidden. Structure published by Sazanov & Hinchcliffe in 2006.