
Головна сторінка Випадкова сторінка
КАТЕГОРІЇ:
АвтомобіліБіологіяБудівництвоВідпочинок і туризмГеографіяДім і садЕкологіяЕкономікаЕлектронікаІноземні мовиІнформатикаІншеІсторіяКультураЛітератураМатематикаМедицинаМеталлургіяМеханікаОсвітаОхорона праціПедагогікаПолітикаПравоПсихологіяРелігіяСоціологіяСпортФізикаФілософіяФінансиХімія
The Light and Dark Reactions
Дата добавления: 2014-11-12; просмотров: 545
|
|
A long series of experiments, dating from the turn of this century, established that the overall process of photosynthesis occurs in two interdependent parts that can be experimentally separated. One part, the light reactions, is directly dependent on light and stops if light energy is unavailable. The second part, the dark reactions, is light independent and may continue in darkness if all of the necessary reactants are present. This was most clearly demonstrated by the experiments of R. Emerson and W. Arnold in the 1930s.
Emerson and Arnold studied photosynthesis in plants exposed to flashes of light lasting only a few milliseconds. They found that a brief flash of intense light could be followed by a period of darkness lasting many times longer without any diminution in the photosynthetic rate. In fact, a period of darkness was required after the bright flash for a given quantity of light to be used most efficiently. Emerson and Arnold interpreted this to mean that two separate reactions occur in photosynthesis in which the products of an initial rapid light reaction are subsequently used in a much slower dark reaction. A cyclic pathway for at least some of the intermediate compounds linking the two pathways was indicated by the fact that the light reactions are greatly inhibited if insufficient time is allowed for completion of the dark reactions between flashes of light. This suggested that the dark reactions convert products of the light reactions into forms in which they are used again in the light reactions.
Later work identified the ADP-ATP couple and NADP as the intermediates linking the light and dark reactions. The immediate products of the light reactions, used as energy sources for the dark reactions, are ATP and reduced NADP (or NADPH). In the dark reactions, these substances are converted to ADP and oxidized NADP, which then cycle back as reactants for the light reactions. Adding these coupling intermediates to Reaction 8-5 gives the new expression for the light reactions:
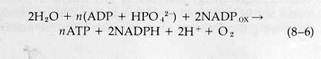
Note that H2O enters the sequence in the light reactions and CO2 in the dark reactions. If like terms are canceled on both sides of Reactions 8-6 and 8-7, Reaction 8-5 is obtained. Only the light reactions are totally unique to photosynthesizing organisms. Most of the dark reactions, which can also produce amino acids or fatty acids and other intermediates in addition to carbohydrates, are common to all living organisms.
Localizing the light and dark reactions in specific parts of chloroplasts was accomplished by R. B. Park and N. G. Pon (1963), who used a centrifuge to separate sonicated chloroplasts into grana and stroma fractions. The grana, which spun down into an insoluble pellet, were found to contain the light-absorbing pigments and the system converting ADP to ATP. The stroma, which remained in suspension in the form of^a colorless solution of proteins, was found to contain the maj^or enzymes of CO2 fixation. These results clearly indicated that the light reactions are concentrated in the grana and the dark reactions in the stroma.
Light Reactions of Photosynthesis
Light and Light Absorption
Visible light is a form of electromagnetic radiation with wavelengths between about 400 and 750 nm. For many purposes, such as a discussion of the path of light rays through lenses, the energy of light can be regarded as radiating in continuous - beams. For a discussion of the absorption of light energy by pigmented molecules, the fact that light energy actually flows in separate, discrete units or packets must be taken into account. This behavior is analogous to an electron beam that, although following a continuous wave path, is formed from particulate electrons. Each electron in the beam represents a separate unit of energy that follows a wave path through space.
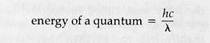
|
| in which h is Planck's constant, c is the velocity of light in a vacuum, and A is the wavelength. Since h and c are constants, |
The individual packets of energy forming a beam of light are called quanta (singular quantum) or photons. Each quantum contains an amount of energy related to its wavelength by the equation:
|
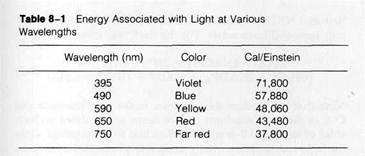
the energy of a quantum increases as the wavelength decreases. For a given wavelength, the energy of a quantum has a constant value. The energy content of light quanta at various wavelengths is given in Table 8-1 in cal/Einstein. The Einstein relates light energy to a gram-molecular weight and is equivalent to a "mole" of light, 6.023 x 1023 quanta. From the table it can be seen that 1 mol of chlorophyll, containing 6.023 x 1023 molecules and absorbing 1 Einstein of red light at 650 nm, wi^l absorb energy equivalent to 43,480 cal.
Molecules such as chlorophyll appear colored because they absorb the energy of light at certain wavelengths and transmit or reflect the energy at other wavelengths. The particular color seen as the molecule's pigment is produced by the transmitted or reflected light. Absorption of light is a property of certain electrons in a molecule that lie either in orbitals surrounding an atomic nucleus or in molecular orbitals associated with more than one atom of the molecule.
Electrons in these orbitals exist at characteristic energy levels. If one of the electrons absorbs the energy of a quantum of light, it undergoes a transition to a new orbital with a higher energy level. In the new orbital, the electron is said to be in an excited state. The difference in energy levels between the unex-cited state (called the ground state) and the excited state has a fixed value for an electron in a given position in a molecule. This value is exactly equivalent to the amount contained in a single quantum of light at a particular wavelength. If the energy of this quantum is absorbed, the electron jumps from the ground to the excited state. All of the energy in a single activating quantum is absorbed; other quanta striking the molecule at different wavelengths have no effect and are transmitted or reflected.
The excited state is so unstable that an electron can persist in an excited orbital for only a fraction of a second. Release from the excited orbital may occur by one of several pathways. The excited electron may simply drop back to its ground state, releasing all of its absorbed energy as heat, or it may release the absorbed energy as both heat and light. The light energy released in the latter case, called fluorescence, is radiated at a slightly longer wavelength and thus represents a quantum containing slightly less energy than the wavelength of the quantum absorbed. The difference between the absorbed and fluoresced quantum is released as heat.
Another pathway for the excited electron has greater significance for photosynthesis. If the excited molecule is situated close enough to another molecule, the orbital occupied by its excited electron may extend outward far enough to overlap orbitals in the second. Transfer of an excited electron to an orbital in the second molecule may then occur, particularly if the orbital taken up in the second molecule represents a ground rather than excited state and is thus stable. Once transferred to a suitable acceptor in this way, the energy of the electron has been "trapped" as chemical energy. Any difference in energy between the excited orbital in the light-absorbing molecule and the stable orbital in the acceptor molecule is released as heat. The energy absorbed by the pigmented molecules active in photosynthesis is converted to chemical energy in this way—by transfer of excited electrons to stable orbitals in acceptor molecules.
Chlorophyll molecules contain an extensive series of electrons capable of absorbing light and jumping to excited orbit-' als, distributed around the porphyrin ring in the shaded regions shown in Fig. 8-11. The multiple electrons and orbitals each absorb light of different quantal energy, thus broadening the distribution of wavelengths absorbed and producing an absorption spectrum with multiple peaks in the form of a smooth curve rather than a single sharp peak (Fig. 8-12). Each of the different chlorophyll molecules, a, b, and c, has a characteristic absorption spectrum determined by the particular distribution of electrons in its porphyrin ring. The distribution is modified by the binding of the different chlorophylls to each other and to proteins, producing" the variations in wavelengths absorbed by the particular chlorophyll types.
During the absorption of light quanta, the electrons in the porphyrin ring of the chlorophylls take up an altered distribution representing the excited state. This state is so unstable that an excited electron is easily lost to an acceptor molecule, greatly facilitating electron transfer and conversion of light to chemical energy.
Light Absorption in Chloroplasts
| <== предыдущая лекция | | | следующая лекция ==> |
| Photosynthesis and the Chloroplast | | | Photosynthetic Units and Light-harvesting Assemblies |