
Ãîëîâíà ñòîð³íêà Âèïàäêîâà ñòîð³íêà
ÊÀÒÅÃÎв¯:
Àâòîìîá³ë³Á³îëîã³ÿÁóä³âíèöòâî³äïî÷èíîê ³ òóðèçìÃåîãðàô³ÿÄ³ì ³ ñàäÅêîëîã³ÿÅêîíîì³êàÅëåêòðîí³êà²íîçåìí³ ìîâè²íôîðìàòèêà²íøå²ñòîð³ÿÊóëüòóðà˳òåðàòóðàÌàòåìàòèêàÌåäèöèíàÌåòàëëóðã³ÿÌåõàí³êàÎñâ³òàÎõîðîíà ïðàö³Ïåäàãîã³êàÏîë³òèêàÏðàâîÏñèõîëîã³ÿÐåë³ã³ÿÑîö³îëîã³ÿÑïîðòÔ³çèêàÔ³ëîñîô³ÿÔ³íàíñèÕ³ì³ÿ
Organization of the Components of Photosystems I and
Äàòà äîáàâëåíèÿ: 2014-11-12; ïðîñìîòðîâ: 582
|
|
IIComplete disruption of inner chloroplast membranes releases both photosystems I and II. Using this technique, J. P. Thornber (reviewed in Thornber, 1975) and others have shown that the photosystems are released as high-molecular-weight
particles containing chlorophyll assemblies in association with reaction centers and some electron-transporting substances. Although the components vary with the particular methods used, an isolated photosystem II particle proves to contain a light-harvesting assembly combined with chlorophyll a and b in approximately equal quantities. Carotenoids are also present in the photosystem II complex, at levels approximating one-third to one-seventh of the amount of chlorophyll. The photosystem II particles also contain the reaction center for this system, chlorophyll P680, and cytochrome b3. Photosystem I particles contain both chlorophyll a and b in a ratio of about 20-30 chlorophyll a molecules to each chlorophyll b molecule. The reaction center chlorophyll of photosystem I, P700, can also be detected in this particle, with about one P700 molecule for every 45 chlorophyll a molecules. Depending on the isolation methods used, the photosystem I particles may also contain cytochrome f, cytochrome b6, plastocyanin, and < ferredoxin.
Complete disruption of inner chloroplast membranes also releases a large chlorophyll-protein particle that contains as much as 40-60% of the total chlorophyll content of chloro-plasts, including both chlorophylls a and b. The complex, now called the light-harvesting chlorophyll-protein or LHCP complex, has no reaction center and is apparently inactive in converting light energy to chemical energy. However, the LHCP complex is believed to act as a large antenna, absorbing and delivering quanta to the photosystems. Because the LHCP complex is most frequently found in close association with photosystem II, it is believed to pass the energy of absorbed quanta primarily to this photosystem.
Arrangement of Photosystems I and II and Electron Transport Carriers within Thylakoid MembranesThe distribution of the components of the Z-pathway within thylakoid membranes has been analyzed by exposing isolated thylakoids to a variety of nonpenetrating agents, in experiments similar to the approach used to work out the topography of mitochon-drial membranes (see p. 159). The agents used (reviewed in DePierre and Ernster, 1977) include antibodies made against individual components, chemicals that label or modify parts of the system exposed at either membrane surface, artificial electron donors or acceptors, and digestion of exposed groups by specific lipase and protease enzymes. Since the agents used do not penetrate across membranes, reaction with intact thylakoids marks only the components of the Z-pathway facing the outer, stromal surface of the thylakoid membranes. Reaction with broken thylakoids marks the components facing the thylakoid compartment as well as the outer surface.
Both photosystems I and II are accessible to antibodies, enzymes, and chemical labels from both sides of thylakoid membranes and thus probably span the entire thylakoid bilayer. CFj, ferredoxin, and ferredoxin-NADP reductase (the flavoprotein linking ferredoxin and NADP) are all accessible to antibodies, enzymes, and markers in unbroken thylakoids,
indicating that these components are located in the bilayer half I1^
facing the stroma. Cytochrome f and plastocyanin react in bro- |1
ken thylakoids, indicating their distribution in the bilayer half I -
facing the thylakoid compartment. Since the plastoquinones J
are unavailable from either side, they are probably completely I
buried within the hydrophobic core of the membrane. These I
results establish that the components of the Z-pathway are I
distributed asymmetrically in thylakoid membranes, in the I
probable arrangement shown in Fig. 8-17.
The Spatial Relationship of Z-Pathway Components to the I Mechanism Synthesizing ATPThe asymmetric location of I the elements of the Z-pathway in thylakoid membranes and the fact that only some of the electron-transporting substances are dual hydrogen-electron carriers provide the basis for ATP synthesis according to the Mitchell hypothesis (see Hinkle and McCarty, 1978, and Mitchell, 1979). In chloroplasts, the first H+ ions added to the gradient are considered to be derived from the reaction splitting H2O, which is proposed to take place on the membrane surface facing the thylakoid compartment. Since chlorophyll P680 is a pure electron carrier, the 2H+ removed from H2O are released to enter the thylakoid com- I partment. The electrons removed from H2O, after excitation in the P680 reaction center, pass from P680 to the plastoquinones J of the primary acceptor pool. Plastoquinones are dual hydrogen-electron carriers, and the 2H+ required for the reduction of a plastoquinone molecule in the pool are derived from the solution on the stroma side of the thylakoid membrane. These H+ ions are expelled into the thylakoid compartment as the electrons pass from the plastoquinone to cytochrome f, a non-H+carrier located on the side of the membrane facing the thylakoid compartment. Mitchell has proposed that a plastoquinone-cytochrome cycle similar to the mitochondrial Q cycle (see pp. 155-156) may also operate at this point in chloroplasts to increase the number of H+ ions expelled across the membrane. From cytochrome f, the electrons pass to plastocyanin and the P700 reaction center of photosystem I. All of the carriers of this segment are non-H+ carriers. After excitation, the electrons pass through the short carrier chain to FAD, a dual H+-electron carrier facing the stroma. Reduction of FAD removes a second pair of H+ ions from the stroma, converting FAD to FADH2. The electrons and one of the 2H+ carried by the FAD are delivered to NADP at the end of the sequence, and the other H+ is released again into the stroma.
The total Z-pathway is thus considered at a minimum to remove 3H+ from the stroma and to expel 4H+ into the thylakoid compartment for each electron pair flowing from H2O to NADP. An additional 2H+ may be transferred from the stroma to the thylakoid compartment if a plastoquinone cycle operates in chloroplast electron transport as proposed by Mitchell. One H+ derived from the stroma remains linked to the NADP reduced in the last step. The H+ gradient created by the mechanism, low in the stroma and high inside the thylakoid compartment, provides the energy that drives ATP

|
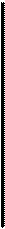 Figure 8-17The asymmetric location of the components of the chloroplast electron transport carriers in thylakoid membranes. The hypothetical flow of electrons (solid lines) and H+ ions (dotted lines) to produce an H+ gradient is shown in the diagram (see text).
Figure 8-17The asymmetric location of the components of the chloroplast electron transport carriers in thylakoid membranes. The hypothetical flow of electrons (solid lines) and H+ ions (dotted lines) to produce an H+ gradient is shown in the diagram (see text).
 synthesis. Extension of the ATPase lollipops into the stroma and the direction of H+ ion expulsion in chloroplasts makes the thylakoid compartment the functional equivalent of the in-termembrane compartment in mitochondria (Fig. 8-18).
synthesis. Extension of the ATPase lollipops into the stroma and the direction of H+ ion expulsion in chloroplasts makes the thylakoid compartment the functional equivalent of the in-termembrane compartment in mitochondria (Fig. 8-18).
The total amount of ATP synthesized for each pair of electrons following the Z-pathway from H2O to NADP, as noted, remains in doubt. In the original experiments carried out by Arnon and his coworkers, apparently, one ATP molecule was synthesized for every electron pair. More recently, this ratio has gradually risen to fractional values between one and two ATP molecules per electron pair with improvements in the procedures used to isolate and maintain chloroplasts (see, for example, Hall et al., 1971). This has prompted the hypothesis that, in intact cells, each electron pair moving through the Z-pathway leads to the synthesis of two ATP molecules.
Thylakoid infrastructure and the Z-Pathway
Although sectioned thylakoid membranes' show evidence of extensive particulate substructure (Fig. 8-19), it has not been possible to relate the image seen in sections to either the photosystems or the electron transport carriers of the light reactions. Somewhat greater success has been achieved by application of the freeze-fracture technique (see the Appendix). This technique, which frequently splits membrane bilayers into inner and outer halves, exposes particles of two sizes inside the thylakoid membranes (Fig. 8-20). The small particles, about 11 nm in diameter, are distributed throughout both grana and stromal lamellae membranes. The large particles, 17

|

|
Figure 8-18The location of the ATPase lollipops in mitochondria (a) and chloroplasts (b) indicates that H+ ions are expelled into the inter-membrane compartment in mitochondria and the thylakoid compartment in chloroplasts.
ran in diameter, are restricted to grana, probably to the membranes in regions where adjacent thylakoids join together.
Tracing the appearance of the 11- and 17-nm particles in developing chloroplasts has revealed the probable relationship of the particles to the two photosystems and the LHCP complex (see Henriques and Park, 1976, and Armond, Staehelin, and Arntzen, 1977). Thylakoids in the chloroplasts of immature leaves contain only the small particles, which are distributed throughout the membranes. Biochemical analysis shows that these thylakoids contain both photosystems but lack the LHCP complex. Upon maturing, the chloroplast inner membranes develop the LHCP complex, which becomes associated with photosystem II. At the same time, the large particles ap-

Figure 8-19Participate substructure visible in the thylakoid membranes of a chloroplast from Aspidistra, x 197,000. FromBiochemistry of Chloroplasts, Vol. 1, ed. T. W. Goodwin, 1966. Courtesy of T. E. Weier and Academic Press, Inc.
I
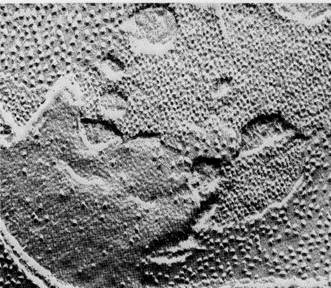
Figure 8-20Freeze-fractured thylakoid membranes of spinach chlo-roplasts. Particles of two distinct sizes are exposed by fractures that split the bilayer halves, x 90,000. From Photophysiology, Vol. 3, ed. A. C. Giese, 1968. Courtesy of D. Branton and Academic Press, Inc.
|
pear in the thylakoids with no increase in total particle number. These results suggest that both photosystems I and II appear as the smaller particles in freeze-fracture preparations when the LHCP complex is not present. When the LHCP complexes appear in the membranes, they associate with photosystem II to form the large particles.
The light reactions, from the initial absorption of light to the production of ATP and reduced NADP (NADPH), provide energy (ATP) and reducing power (reduced NADP) for the dark reactions. ATP and reduced NADP accumulate after illumination of chloroplasts; unless they are used within a short time by the dark reactions, with the regeneration of oxidized NADP and ADP, the efficiency of photosynthesis falls.
Dark Reactions: CO2 Fixation in Photosynthesis
The Calvin Cycle
In the dark reactions, chemical energy produced in the light reactions is used to fix CO2 into carbohydrates and a variety of other organic products. Although termed the dark reactions because they do not depend directly on light, these interactions actually take place primarily in the daytime, when ATP and reduced NADP are readily available from the light reactions.
Little progress was made in unraveling the dark reactions until the 1940s, when radioactive tracers first became available to biochemists. One substance in particular, CO2 labeled with the radioactive isotope 14C, made possible the first real breakthroughs in this research.
M. Calvin, A. A. Benson, and their colleagues used this radioactive form of CO2 to trace out the biochemical pathways of the dark reactions. In their experiments, Calvin and his colleagues allowed photosynthesis to proceed in Chlorella in the presence of radioactive CO2. At various times after exposure to the labeled CO2, extracts of carbohydrates and other substances were made from the cells.
If the carbohydrate extracts were made within a few seconds after exposure, most of the radioactivity could be identified with the three-carbon sugar 3-phosphoglyceraldehyde (3-PGAL). The CO2 taken in by Chlorella was incorporated very rapidly into this three-carbon substance, evidently one of the earliest products of photosynthesis. If extracts were made after longer periods, radioactive label showed up in more complex substances, including a variety of six-carbon sugars, sucrose, and starch.
In other experiments, Calvin and his colleagues reduced the amount of CO2 so that photosynthesis could proceed only very slowly, even though adequate light was supplied. Under
Figure 8-21The overall reactants and products of the Calvin cycle.
these conditions, a nonradioactive five-carbon sugar, ribulose-1,5-diphosphate (RuDP), accumulated in quantity in the chloroplasts, suggesting that this substance is the first to react with CO2 in the dark reactions. By similar methods, most of the remaining intermediate compounds appearing between CO2 and six-carbon sugars were identified.
Using this information, Calvin, with his colleagues Benson and J. A. Bassham, were able to piece together the dark reactions of photosynthesis. The cycle is now called the Calvin cycle, or the C3 cycle, because the first sugar product of the cycle is a triose or three-carbon sugar. Calvin was awarded the Nobel Prize in 1961 for his brilliant work in deducing the reactions of the cycle.
Reactions of the Calvin CycleThe reactions worked out by Calvin and his colleagues resemble the Krebs cycle in that the intermediate compounds of the sequence are continuously regenerated as the cycle turns. The cycle uses CO2, ATP, and reduced NADP as net reactants and releases ADP, oxidized NADP, and carbohydrates in the form of 3-PGAL as net products (Fig. 8-21).
In the first reaction of the cycle (Fig. 8-22), CO2 combines directly with the five-carbon, two-phosphate substance RuDP. The reaction, catalyzed by the enzyme ribulose-l,5-diphosphate carboxylase (RuDP carboxylase), produces two molecules of 3-phosphoglyceric acid, a three-carbon- substance. One of these contains the newly incorporated CO2 in the position marked by an asterisk in Fig. 8-22. The reaction requires no input of energy because the two three-carbon products exist at a much lower energy level than RuDP, which can be considered as a high-energy substance. The RuDP carboxylase enzyme catalyzing this reaction makes up as much as 25-50 % of the total protein of chloroplasts. This large quantity is attributed to the

|
fact that the enzyme reacts only relatively slowly with CO2 and thus has a slow turnover time in the reaction. The high content of the carboxylase in chloroplasts evidently compensates effectively for the low reactivity of the enzyme.
The next two reactions of the cycle, which proceed at the expense of ATP and reduced NADP, yield the net carbohydrate product of the cycle, 3-PGAL. In the first of these reactions, 3-phosphoglyceric acid is phosphorylated to form the more reactive substance 1,3-diphosphoglyceric acid. The phosphate added in the reaction is derived from ATP, which is converted to ADP in the process. This activated molecule accepts an H+ ion and two electrons from NADP in the next reaction, a reduction. One phosphate is removed from the substrate in the reaction, which yields 3-PGAL. For each molecule of CO2 attached to RuDP, two molecules of 3-PGAL are ultimately produced. The total reaction sequence to this point uses two ATP molecules and two reduced NADP molecules:

Some of the 3-PGAL produced at this step is required to regenerate the RuDP used in the first step in the cycle; some is released as a surplus to the cycle to enter the major biochemical pathways leading to glucose, sucrose, starch, and other complex products of photosynthesis.
Regeneration of RuDP occurs through a complex of reactions (not shown in Fig. 8-22) that yields as an initial product the five-carbon, one-phosphate sugar ribulose-5-phosphate. This product is then phosphorylated to RuDP at the expense of one additional ATP. This reaction regenerates the RuDP used in the first step in the cycle, and the entire series is ready to turn again.
Three turns of the Calvin cycle are required to yield one molecule of 3-PGAL as a surplus that can be used for synthesis of more complex carbohydrates. In three turns of the cycle through reaction 3 in Fig. 8-22, six molecules of 3-PGAL are formed (for a total of 18 carbons). Five of these molecules (containing 15 carbons) enter the complex series that regenerates the three RuDP molecules used in the three turns. The remaining molecule of 3-PGAL is surplus and can enter synthetic

|
Figure 8-23A summary of the Calvin cycle, showing only carbon chains and phosphate groups.
pathways forming more complex substances. A single turn of the cycle can thus be regarded as taking up one molecule of CO2 and yielding one unit of carbohydrate, (CH2O). Six turns of the cycle are required to make enough carbohydrate units to yield one molecule of a hexose such as glucose.
For each turn of the cycle, a total of two ATP and two reduced NADP molecules are used in steps 2 and 3 in Fig. 8-22. One additional ATP molecule enters the cycle in the reaction regenerating RuDP, for a total of three ATP molecules for each turn. As net reactants and products, one complete turn of the cycle therefore includes:

Fig. 8-23 summarizes the reactions of the cyclein simplified form.
Formation of Complex Sugars and Starch from 3-PGALThe
primary product of the Calvin cycle, 3-PGAL, is the starting point for synthesis of a variety of complex carbohydrates and
polysaccharides. Glucose and other hexoses are formed through a series of reactions that essentially reverse part of glycolysis (see Fig.'7-4). In glycolysis, two reactions between glucose and 3-PGAL are essentially irreversible. These reactions are run in the reverse direction, synthesizing glucose from 3-PGAL, by the activity of enzymes that replace the gly^lytic enzymes at these points.
Note from Fig. 7-4 that no extra ATP is required to synthesize glucose from two molecules of 3-PGAL. Sufficient energy is contained in 3-PGAL to drive the reaction sequence in reverse when the triose sugars are present in high concentration. And, although inorganic phosphate is released as a part of the sequence from 3-PGAL to glucose, none is incorporated into ATP. Thus, there is no net change in the amount of ATP in the synthesis of glucose from 3-PGAL.
Free glucose is actually formed in only very limited quantities in the chloroplasts of most plants. Instead, glucose-1-phosphate and other hexose-1-phosphates are used as the starting points for synthesis of sucrose, starch, cellulose, and a wide variety of additional organic molecules. In addition to carbohydrates, amino acids and proteins also become labeled rapidly in illuminated chloroplasts supplied with radioactive CO2. In fact, label frequently shows up in amino acids before it

|
Figure 8-24Integration of the C4 and Calvin cycles (see text).
appears in the more complex carbohydrates, suggesting that amino acids are probably synthesized by transamination (see also p. 160) of intermediates falling in the sequence between CO2 reduction and glucose synthesis. All of the amino acids required for protein synthesis can be synthesized by most plants, either inside chloroplasts or in the surrounding cytoplasm, by pathways starting from products of the dark reactions. Protein synthesis, occurring on ribosomes suspended in the chloroplast stroma, can also be detected inside chloroplasts, along with polymerization of nucleotides into DNA and RNA (see Supplements 12-2 and 13-1).
After a few minutes of photosynthesis in labeled CO2, lipids within chloroplasts also become labeled. In some cases, synthesis of labeled lipids, including fatty acids, fats, and galactolipids, may represent as much as 30% of the total labeled material inside chloroplasts after 1-2 min of photosy#-thesis. This lipid synthesis includes the various photosynthetic pigments: Chloroplasts are able to carry out all of the reactions required to make chlorophylls and carotenoids from simple precursors originating from the dark reactions of the Calvin cycle.
The balance among carbohydrates, fats, and amino acids synthesized in chloroplasts varies widely among different species of plants. In some, nearly all of the CO2 absorbed is incorporated into carbohydrates such as sucrose, formed from glucose and fructose. In others, such as the alga Chlorella, the synthesis of fats and amino acids greatly exceeds sucrose synthesis, which may account for 5% or less of the CO2 absorbed. In any event, the chloroplasts of most species contain the enzymes and intermediates required for synthesis of a wide variety of substances in addition to carbohydrates and indeed have synthetic capacity practically equivalent to entire cells.
The C4 Cycle: An Important Supplement to the Calvin Cycle
During the 1960s, several groups working independently, including H. P. Kortschak and his colleagues (Kortschak, Hartt, and Burr, 1965) and M. D. Hatch and C. R. Slack (1966), discovered that certain plants are able to carry out a supplemental series of reactions that apparently improves the efficiency of CO2 utilization by the Calvin cycle. This pathway, now called the C4 cycle, was discovered when the Kortschak group and Hatch and Slack looked for the earliest-labeled intermediates produced in corn, sugar cane, and other tropical grasses after the plants were exposed to labeled CO2. Surprisingly, the earliest label appeared in a pool of malic and other four-carbon acids instead of 3-PGAL as in the Calvin cycle. Intermediates of the Calvin cycle were also found to be labeled a few seconds after the appearance of label in the four-carbon acids.

|
| The product of this carboxylation follows a subsequent pathway that varies among different plants using the C4 cycle. |
Hatch and Slack proposed that the initial appearance of label in the pool of four-carbon acids and in other intermediates was part of a side cycle linked to the main Calvin cycle (Fig. 8-24). The main feature of their proposed cycle, which has been supported by extensive experimentation, is initial incorporation of CO2 into organic substances by combination with an activated product of pyruvic acid, phosphoenol pyruvic add:


|
Figure 8-25Organization of the C4 and Calvin cycles in mesophyll and bundle sheath cells of grasses (see text). Modified from an original courtesy of M. D. Hatch. © Academic Press, Inc., from Current Topics in Cellular Regulation 14 : 1 (1978).
In the most frequently observed route, oxaloacetic acid is next reduced to malic acid in a reaction that uses NADP as an electron donor:

The malic acid produced apparently delivers CO2 to the Calvin cycle. In the vicinity of the Calvin cycle, malic acid is oxidized by the enzyme malic add dehydrogenase, which decar-boxylates the acid at the same time:

This step replaces the NADPred used earlier in the Cj pathway. The CCh released enters the Calvin cycle by the regular route by combination with RuDP, and the Calvin cycle turns as usual. The other product of this reaction, pyruvic acid, is phosphorylated at the expense of ATP to replace the phos-phoenol pyruvic acid used in the first step of the C4 cycle.
At first glance the C4 pathway appears to be a "futile" cycle that results only in the net breakdown of ATP. However, it evidently increases the availability of CO2 to the Calvin cycle by compensating for what appears to be an evolutionary im-
perfection in the activity of RuDP carboxylase, the enzyme catalyzing the first uptake of CO2 in the Calvin cycle. Recently, oxygen has been shown to compete effectively with CO2 for the active site on RuDP carboxylase, diverting the enzyme from its central role in the Calvin cycle to activity as an oxygenase (see Bahr and Jensen, 1974). The products of the oxygenation of RuDP by the enzyme enter the process of photorespiration in peroxisomes and are lost to the Calvin cycle (see Supplement 7-3). Plants with the C4 cycle, such as grasses, are able to get around this deficiency in RuDP carboxylase through the following compensating mechanism.
In grasses, the C4 cycle occurs in the chloroplasts of mesophyll cells that lie close to the external surface of the plants (Fig. 8-25). Because the cells are near the plant surface, they contain oxygen in relatively high concentration. However, these cells lack RuDP carboxylase, effectively preventing diversion of carbohydrate intermediates to the photorespiration pathway through activity of the enzyme as an oxygenase. Instead, CO2 is taken up in the C4 cycle, leading to an extensive pool of four-carbon acids. Malic acid from these cells diffuses to deeper tissues of the plant to a cell type called bundle sheath cells. In these cells, malic acid is oxidized, releasing CO2 in quantity. The chloroplasts of these cells contain RuDP carboxylase in normal amounts. Since oxygen is present in reduced concentration in the deeper layers, the RuDP car-
boxylase effectively carries out its Calvin cycle function of CO2 fixation, without diversion to its alternate oxygenase role. The result is much greater efficiency in the activity of RuDP car-boxylase in CO2 fixation and, consequently, much greater efficiency in photosynthesis. Under optimum conditions, the plants with the C4 cycle carry out photosynthesis at about twice the rate of plants without it. Interestingly, in the bundle sheath cells of some plants with the C4 pathway, thylakoids occur in chloroplasts in single, extended form as well as in grana stacks. The relationship of this unusual thylakoid arrangement to the pattern of photosynthesis in the bundle sheath cells is unknown.
Plants with the C4 cycle are found primarily in tropical and subtropical regions, particularly in more arid habitats. Water loss is reduced in C4 plants through a side effect on the stomata, the minute openings in leaves that admit CO2 and allow water vapor to escape. The greater efficiency in CO2 uptake reduces the size of these openings and the amount of H2O lost by transpiration through them. As a result, C4 plants are about twice as economical in water use as other plants. Among the plants utilizing the Q pathway are the important crop grasses corn, sugar cane, and sorghum.
Integration of Chloroplast Activity in Plant Cells
Most of the carbohydrate exported from chloroplasts to the cytoplasm is transported through the chloroplast membranes in the form of dihydroxyacetone phosphate, a three-carbon substance that is readily interconverted with 3-PGAL (see reaction 4, Fig. 7-4). In the cytoplasm, dihydroxyacetone phosphate is converted into 3-PGAL, which may serve as the starting point for carbohydrate synthesis through reversal of the first half of the glycolytic sequence. Alternatively, 3-PGAL may be oxidized in the second half of glycolysis, producing pyruvic acid that subsequently enters mitochondria for complete oxidation to CO2 and H2O. Dihydroxyacetone phosphate is evidently transported across chloroplast membranes by a transport protein that exchanges an inorgsStic phosphate group, moved from outside to inside the chloroplast, for each molecule of dihydroxyacetone phosphate moved outside (see Flugge and Heldt, 1976).
Chloroplast membranes also contain transport proteins specific in their activity for inorganic phosphate alone, some ribose sugars, and dicarboxylic acids (see, for example, Wang and Nobel, 1971, and Heldt and Rapley, 1970). The dicarboxylic acids transported include malic, succinic, fumaric, oxalo-acetic, and alpha-ketoglutaric acids, as well as two amino adds, aspartic and glutamic. In plants with the Q cycle, pyruvic and phosphoenolpyruvic acids are also transported across the chloroplast envelopes. Mitochondrial and chloroplast activity in C4 plants is integrated through the system
transporting dicarboxylic acids across chloroplast membranes. Two steps of the C4 cycle, reduction of oxaloacetic acid and oxidation of malic acid, occur inside mitochondria.
Whether the ATP synthesized inside chloroplasts can be directly utilized for cell activities in the surrounding cytoplasm has not been determined conclusively. Although a transport system exchanging ADP for ATP has been detected in the chloroplasts of a few species (see, for example Heldt, 1969), most of the available evidence indicates that chloroplast membranes in most plants are impermeable to the ADP/ATP couple. If so, ATP for cytoplasmic activities cannot be obtained directly from the light reactions in most plants. However, since dihydroxyacetone phosphate can enter the cytoplasm for oxidation, the reactions inside chloroplasts may lead indirectly to the synthesis of large quantities of ATP in the cytoplasm. Chloroplasts in all species seem to be impermeable to NADP.
The diversion of carbohydrates into oxidative pathways through the alternate activity of RuDP carboxylase as an oxygenase has already been mentioned. In some plants as much as 50% of the CO2 fixed in the Calvin cycle may be oxidized by this route.
Through the various synthetic routes linked directly or indirectly to chloroplasts, plant cells are able to synthesize all of the complex organic materials required for life from simple inorganic precursors. The oxidative routes linked to chloroplast activity supply all of the chemical energy required for this synthesis. The total system operates with such efficiency that not just plants but most living organisms of the earth are supported by the light energy captured and converted into chemical energy inside chloroplasts.
Photosynthesis in Prokaryotes
The photosynthetic mechanisms of the two prokaryotic groups differ fundamentally. The photosynthetic bacteria, the green and purple bacteria, possess a comparatively primitive system limited essentially to the activities carried out by photosystem I in the eukaryotic plants. Lacking photosystem II, the photosynthetic bacteria cannot utilize H2O as an electron donor and do not evolve oxygen in photosynthesis. In contrast, the blue-green algae, although typically prokaryotic in cellular organization, carry out photosynthesis by essentially the same mechanisms as higher plants. Since photosystems I and II are present, H2O can be used as an electron donor, and oxygen is evolved in photosynthesis. As far as has been determined, the molecular components and reaction sequences of photosynthesis in blue-green algae are identical to those in the eukaryotic algae and higher plants.
The photosynthetic bacteria differ from the remaining photosynthetic organisms of the world primarily in the photo-systems and light reactions. All photosynthetic groups, prokaryotic and eukaryotic, use the Calvin cycle to fix CO2 and carry out the dark reactions in a similar way.
|

Figure 8-26Bacteriochlorophyll (BChl). In BChl a, X = —C2H5; an additional —H occurs at the 4-carbon in BChl a. In BChl b, X = =CH—CH3.
The Photosynthetic Bacteria
The green and purple photosynthetic bacteria are small taxonomic groups that together comprise less than 50 known species. These bacteria utilize a distinct family of chlorophylls, the bacteriochlorophylls (BChls; Fig. 8-26), which absorb light most strongly in the far red wavelengths and transmit almost all of the visible wavelengths. As a result, the BChls contribute little distinctive color to either bacterial group. Instead, the colors of these bacteria come from different carotenoids occurring as accessory pigments in association with the BChls.
At least five types of BChl, a, b, c, d, and e, are present in different bacteria. Purple bacteria contain BChl a and b. BChl c, d, and e predominate in the green bacteria in combination with small quantities of BChl a. A form of BChl a absorbing light at 870 nm, ^§lled P870, apparently serves as the reaction center in all bacteria.
The photosynthetic units carrying out the light reactions are tightly bound to membranes in bacteria, as they are in chloroplasts. The bacterial units, first isolated by D. W. Reed and R. K. Clayton (1968), contain a light-harvesting assembly of about 50 BChl molecules serving a P870 reaction center. The reaction center contains four P870 molecules, two of which interact closely to trap and release excited electrons (see McEl-roy, Feher, and Mauzerall, 1972). Quinones and cytochromes ; of the c type are usually linked to the bacterial photosystem particles. Other cytochromes and quinones are also present as electron carriers in bacterial membranes without tight binding
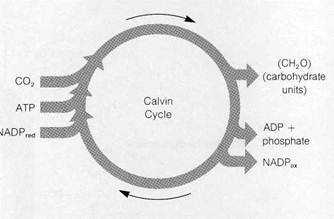
Figure 8-27Noncyclic photosynthesis in bacteria (see text).
to the photosynthetic units.
The bacterial photosystem and the electron carriers function in a manner resembling photosystem I in eukaryotes. Both cyclic and noncyclic electron flow can be detected. In noncyclic flow electrons are accepted by the photosystem from substances such as H2S, H2, thiosulfate, and various organic molecules (Fig. 8-27 shows a noncyclic system based on H2S). All of these usable donors release electrons at relatively high energy levels when oxidized; the electrons released from water exist at energy levels too low to enter the system. From the donor, the electrons flow through a chain of carriers to reach the reaction center, where they are raised to high energy levels through the absorbance of light energy. The electrons then pass to a primary acceptor and, after traversing a short chain, reduce the final acceptor of the chain, which in bacteria is NAD instead of NADP. Cyclic photosynthesis (Fig. 8-28) proceeds essentially as it does in eukaryotes. Electrons raised to high energy levels in the P870 reaction center flow through a series of carriers to reach the same reaction center again with a net synthesis of ATP.
Light-induced electron flow through the bacterial photosystem and carriers builds up an H+ gradient across the membrane containing the system. If the systems are isolated in closed vesicles containing the bacterial-membrane-bound ATPase, both cyclic and noncyclic flows build up an H+ gradient sufficient to drive ATP synthesis.
The electron carriers acting in the bacterial photosystem have proved difficult to identify. Bacterial membranes contain a variety of b and c cytochromes, along with cytochromes of
 other types, that absorb light so similarly that clear, nonover-lapping spectra cannot be obtained. The situation is further complicated by the fact that many of the carriers in bacterial membranes apparently transport high-energy electrons originating from both photosynthesis and respiration. Within the margin of uncertainty created by these difficulties, the usual battery of techniques used for identifying and sequencing the carriers (see pp. 149-150) indicates the provisional systems shown in Figs. 8-27 and 8-28.
other types, that absorb light so similarly that clear, nonover-lapping spectra cannot be obtained. The situation is further complicated by the fact that many of the carriers in bacterial membranes apparently transport high-energy electrons originating from both photosynthesis and respiration. Within the margin of uncertainty created by these difficulties, the usual battery of techniques used for identifying and sequencing the carriers (see pp. 149-150) indicates the provisional systems shown in Figs. 8-27 and 8-28.
The carriers are believed to act in electron transport as follows. In noncyclic photosynthesis (see Fig. 8-27), electrons from a high-energy donor such as H2S are believed to flow through a chain of carriers in the sequence coenzyme Q —> cytochrome b —> cytochrome c. The varieties of cytochrome b and c present, which differ extensively among the photo-synthetic bacteria, have no exact counterparts in eukaryotes. The passage of each electron pair through this chain generates an H+ gradient that drives synthesis of at least one ATP molecule. From cytochrome c, electrons pass to P870 at the reaction center, where they are raised to higher energy levels through the energy of absorbed light. From P870 the excited electrons pass to the primary acceptor.
Recent research with techniques designed to detect extremely fast spectroscopic changes have given some clues to the identity of the primary acceptor in bacteria. Using these new techniques, which can detect changes with a duration of as little as lfT11 s, P. L. Dutton (see Dutton et al., 1976) identified a substance called bacteriopheophytin as the primary acceptor. Bacteriopheophytin is essentially identical to bac-teriochlorophyll, except that H+ ions occupy the center of the porphyrin ring instead of Mg2"1^. From the primary acceptor, the electrons pass to NAD, probably through at least one other unidentified carrier. The reduced NAD may be used indirectly as a source of reducing power for the dark reactions or may pass its electrons to the respiratory electron chain (see Fig. 7-28), leading to synthesis of additional ATP.
Cyclic electron flow (see Fig. 8-28) is believed to include the same coenzyme Q —> cytochrome b —» cytochrome c sequence of carriers, acting in such a way that high-energy electrons flow to the sequence from the primary acceptor. In this case, the research of Dutton and others indicates that the electrons pass from the primary acceptor to a quinone molecule complexed with iron, identified as "Q/Fe" in Fig. 8-28. From here, the electrons flow to uncomplexed coenzyme Q and the cytochrome chain, finally reaching ground state orbitals in P870 again. As in eukaryotes, this cyclic electron flow generates ATP.
In most photosynthetic bacteria, the membranes bearing the photosystems and their associated carriers are suspended in the cytoplasm as closed spherical or flattened sacs (Figs. , 8-29 and 8-30). Whether the sacs float freely in the cytoplasm or retain connections to the plasma membrane is a matter of ■ some debate. In a few photosynthetic bacteria, the photosys-tem and electron carriers are concentrated in the plasma membrane and no cytoplasmic sacs are evident.

Figure 8-28Cyclic photophosphorylation (see text).
One bacterial group, the genus Halobacterium, has a primitive photosynthetic mechanism that differs radically from any yet described. The system in these bacteria, investigated by E. Racker and W. Stoeckenius (1974), provides some of the strongest evidence supporting Mitchell's chemiosmotic hypothesis (see also pp. 156-158). The photosynthetic membranes of Halobacterium contain only a single pigmented molecule, bacteriorhodopsin, that responds to light by expelling H+ ions across the membrane; no other light-absorbing elements or electron carriers are present. The H+ gradient drives ATP synthesis by a membrane-bound ATPase complex. Racker and Stoeckenius were successful in isolating and purifying the bacteriorhodopsin molecule, which, when added to closed phospholipid vesicles and illuminated, created an H+ gradient across the vesicle membranes. Addition of the ATPase complex allowed the system to synthesize ATP in response to the gradient, clearly supporting the basic tenets of the Mitchell hypothesis.
| <== ïðåäûäóùàÿ ëåêöèÿ | | | ñëåäóþùàÿ ëåêöèÿ ==> |
| Photosynthetic Units and Light-harvesting Assemblies | | | The Blue-green Algae |