
Головна сторінка Випадкова сторінка
КАТЕГОРІЇ:
АвтомобіліБіологіяБудівництвоВідпочинок і туризмГеографіяДім і садЕкологіяЕкономікаЕлектронікаІноземні мовиІнформатикаІншеІсторіяКультураЛітератураМатематикаМедицинаМеталлургіяМеханікаОсвітаОхорона праціПедагогікаПолітикаПравоПсихологіяРелігіяСоціологіяСпортФізикаФілософіяФінансиХімія
Photosynthetic Units and Light-harvesting Assemblies
Дата добавления: 2014-11-12; просмотров: 557
|
|
Emerson and Arnold's research leading to the discovery that two sets of reactions occur in photosynthesis, the light and dark reactions, also gave indications that the molecules absorbing light in chloroplasts act in groups or assemblies rather than singly. This was deduced from experiments in which the amount of light given to their experimental cells in brief, saturating flashes was just sufficient to cause the dark reactions to run continuously at a maximum rate. Under these conditions, the largest possible fraction of the chlorophyll molecules present in the cell was being fully utilized. Light energy absorbed by any additional molecules could not be converted and used by the dark reactions. By comparing the amounts of O2 released with the number of
|
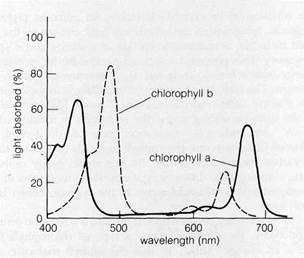
Figure 8-12Absorption spectra of chlorophylls a and b.
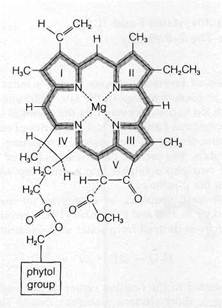
Figure 8-11Distribution of the light-absorbing orbitals in chlorophyll
(see text). *"
chlorophyll molecules per cell in the alga used in their experiments (Chlorella), Emerson and Arnold determined that one O2 molecule was released for 2500 chlorophyll molecules per flash. The subsequent dark reactions also reduced one molecule of CO2 to (CH2O) for each 2500 chlorophyll molecules.
Since it seemed unlikely that each of the 2500 chlorophyll molecules absorbed a quantum of light to drive the release of one O2 molecule, Emerson and Arnold and others proposed that the chlorophyll molecules in a chloroplast are organized into smaller groups called light-harvesting assemblies. A quantum of light absorbed anywhere in the assembly is conducted to sites termed reaction centers, where the conversion of light to chemical energy actually occurs.
The number of light-harvesting assemblies and reaction centers acting in each group of 2500 chlorophyll molecules was considered to depend on the number of quanta needed for the evolution of one molecule of O2. While the number of light quanta required to produce one O2 molecule has not been definitely established, it probably approaches a total of eight (see p. 180). Transformation of each of these quanta by separate light-harvesting assemblies and reaction centers within the 2500 chlorophyll molecules would indicate that an individual assembly in Chlorella contains 2500/8 or about 300 chlorophyll molecules. This hypothetical light-harvesting assembly of —300 chlorophyll molecules, with its reaction center, was termed a photosynthetic unit. Recent isolation of stable particles from chloroplasts of different species containing 200-300 chlorophyll molecules in combination with proteins and a reaction center (see pp. 181-182) indicates that the light-
absorbing pigments are actually organized into photosynthetic units of this type.
The chlorophyll molecules of the photosynthetic units in eukaryotic plants contain both chlorophylls a and b. Of these two chlorophylls, a considerable body of evidence indicates that the reaction centers are based on one or more molecules of chlorophyll a. The light-harvesting assemblies also contain carotenoid pigments, which are capable of passing on the energy of absorbed light to chlorophyll. From estimates of the total number of carotenoid molecules per quantum absorbed in chloroplasts and an analysis of isolated photosynthetic units, the light-harvesting assemblies associated with single reaction centers appear to contain from 50 to 200 carotenoids in addition to the 200-300 chlorophyll molecules present.
Transfer of Absorbed Energy to Reaction CentersHow is
light energy absorbed by a molecule somewhere in a light-harvesting assembly transferred to the reaction center? That such transfer can actually occur is easily demonstrated in a model system consisting of a solution of two different pig-mented molecules that absorb light at distinct wavelengths. If such a solution is irradiated by light at the shorter wavelength absorbed by one of the molecules, both pigments will quickly fluoresce, each at its characteristic wavelength. Since the light is supplied only at the wavelength absorbed by one of the molecules, the absorbed energy must t>e transferred in some manner to the second pigment.
According to the most widely accepted explanation, the absorbing molecule sets up an electromagnetic field through
the vibration of its excited electrons. An adjacent pigment molecule, lying within the vibrational field created by the excited molecule, is induced to vibrate or resonate at the same frequency. This phenomenon has a parallel in the transfer of energy from a broadcasting television antenna to a receiving antenna. The transfer of energy from molecule to molecule in this manner, called inductive resonance, can be highly efficient. In the light-harvesting units, the excitation is considered to "walk" by inductive resonance from a carotenoid to chlorophyll or from one chlorophyll molecule to another until it reaches the reaction center. By this route, many units of energy, arising from different light-absorbing molecules of the photosynthetic unit, could arrive at the reaction center in a short time.
How is energy trapped once it reaches the reaction center? Supposedly, the center contains a type of chlorophyll that forms an energy "sink." In this chlorophyll molecule, the energy level of electrons in the excited state is slightly less than that of the electrons in the other chlorophyll molecules of the photosynthetic unit. Although sufficient energy is supplied from the transferring molecules to raise an electron in the trapping molecule to its excited state, the reverse flow is not likely, because not enough energy is released by the trapping \ molecule to excite the transferring molecules. The energy consequently remains with the reaction center chlorophyll to be released either as heat or long-wave fluorescence, or to be passed on as chemical energy to the initial electron acceptors of photosynthesis.
Photosystems I and IIOriginally the light reactions were believed to be parts of a pathway linked to the absorption of light energy by single chlorophyll reaction centers. Evidence to the contrary came in the late 1950s, when R. Emerson and his coworkers discovered that the rate of photosynthesis in red light is greatly enhanced if light is supplied at two separate wavelengths. Most significant was the finding that the enhancement was retained if the two long wavelengths of light were flashed alternately at intervals of up to several seconds. To Emerson and another investigator, L. N. M. Duysens, this suggested that two light-absorbing systems operate in photosynthesis and that the energy absorbed by one of the systems . is somehow added to the energy absorbed by the other. The relatively long time interval permissible between flashes at the two wavelengths indicated that the linkage between the two systems is in the form of chemical intermediates rather than direct transfer of the excited state. This is because the energy trapped in the excited state is so unstable that return to the ground state, with fluorescence and heat, occurs within fractions of a second unless the high-energy electrons are transferred to stable orbitals in a suitable chemical acceptor. However, the two wavelengths in the experiments were still found to have additive effects even if the delay between them was extended to several seconds. Duysens called the two separate light-absorbing systems photosystem I and photosystem II.
How Photosystems I and II Are Linked: The Z-Pathway
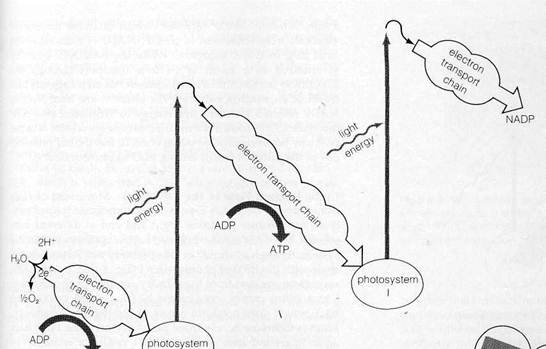
Publication of Emerson's results caused a burst of intensive research in photosynthesis in the late 1950s and early 1960s. This-work led to the discovery that the chemical systems linking photosystems I and II consist of a series of electron transport chains similar to the system conducting electrons in mitochondria. The carriers of the chains proved to be linked with the two photosystems into a multistep sequence now known as the Z-pathway.
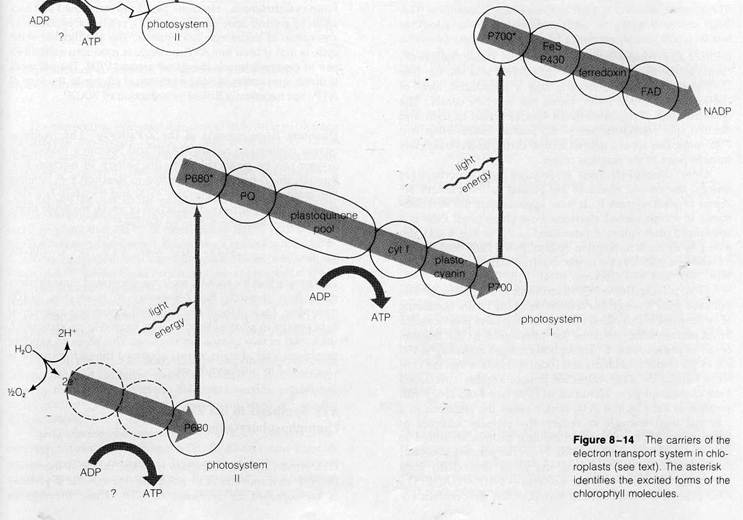
The overall sequence of events in the pathway, first suggested by R. Hill and F. Bendall (1960), are shown in Fig. 8-13. Electrons derived from water in the reaction
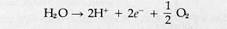
are transferred to the reaction center of photosystem II after traversing a short electron transport chain. The electrons, which at this point are at a relatively low energy level, enter the ground state orbitals in the chlorophyll molecules at the reaction center. The light-harvesting assembly of photosystem II then absorbs light and transfers energy to the reaction center, raising the electrons derived from water to excited orbitals. The excited electrons are transferred immediately to the first acceptor of photosystem II, known as the primary acceptor. From this point they flow through an extended electron transport chain, gradually losing energy until they reach the reaction center of photosystem I. After excitation in photosystem I, the electrons are transferred to the primary acceptor of this photosystem and travel through a short electron transport chain to reach NADP. Electron flow through the system is tightly coupled, as in mitochondria, to ATP synthesis. Thus, the overall reactions of the Z-pathway yield the primary products of the light reactions of photosynthesis, ATP and reduced NADP, and release oxygen as a by-product.
The known electron carriers of the Z-pathway include flavoproteins, iron-sulfur proteins, quinones, cytochromes, and a copper-containing protein unique to chloroplasts, plas-tocyanin. Figure 8-14 shows the positions of the individual carriers as far as they are known. Following the course of electrons through the pathway will show how the photosystems and electron transport chains are considered to work in detail. The first steps in the sequence are only very incompletely understood. Little is known about the reaction splitting water except that it requires the presence of intact thylakoid membranes and manganese (Mn2+) ions. Similarly, the carriers conducting electrons from the H2O to the reaction center of photosystem II have not yet been identified or sequenced.
As the electrons pass to photosystem II for excitation, they reduce the chlorophyll molecules forming the reaction center of this photosystem. The specific chlorophyll at this reaction center was identified by the detection of very brief changes in light absorption occurring as the center was alternately
|
|
Figure 8-13Flow of electrons through the Z-pathway (see text).
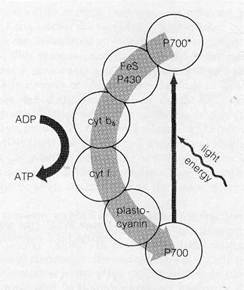
Figure 8—15Cyclic electron flow around P700 (see text).
oxidized and reduced during electron flow. These changes occurred in the light absorbed by chlorophyll a at 680 nm (see Do'ring et al., 1969), indicating that a specialized form of chlorophyll a, called P680, forms the reaction center. The characteristics of the absorbance spectra noted in light and electron spin resonance (see p. 62) studies indicate that two P680 molecules act as a unit to accept, excite, and release electrons in pairs at the reaction center.
Once chlorophyll P680 is reduced and absorbs light energy, the excited electrons are passed to the primary acceptor of photosystem II. It now appears that the first substance to accept excited electrons from chlorophyll P680 is a specialized plastoquinone (identified as PQ in Fig. 8-14), detected by its rapid absorption change as the P680 center loses its electrons and returns to the oxidized state (see Sriehl and Witt, 1969, and Van Gorkom, 1974).
From plastoquinone PQ, electrons flow to a plastoquinone pool and then through the remainder of the chain, consisting of cytochrome f (also called cytochrome b533) and plastocyanin. From plastocyanin, electrons are transferred to the reaction center of photosystem I. Absorption changes occurring at 700 . nm as the center is oxidized and reduced indicate that the center is formed by a two-molecule unit of another specialized form of chlorophyll a, identified as P700 (see Kok, 1961). Absorption of light by the P700 center raises the electrons to a potential high enough to reduce the primary acceptor of photosystem I, probably an iron-sulfur protein identified as P430 (for example, see Ke, 1973). From the primary acceptor, the electrons then flow along the short final chain from ferredoxin (another iron-sulfur protein) —> FAD —» NADP. The FAD present is the prosthetic group of the flavoprotein en-
zyme, ferredoxin-NADP reductase, which catalyzes transfer of electrons from ferredoxin to NADP. NADP is reduced at the final step in the Z sequence. While the total ATP quantity synthesized as a result of electron transport through the -pathway is still controversial, current research suggests that travel of an electron pair over the entire route from H2O to NADP releases sufficient free energy to synthesize two ATP molecules. One known site of sufficient energy release is in the pathway between photosystems I and II; the second probably lies in the initial segment linking H2O to photosystem II.
Cyclic Electron Flow in the Z-PathwayMovement of elec- trons through the entire Z-pathway is sometimes termed nm- cyclic flow because electrons begin and end at different sub- stances. Electrons are also believed to flow cyclically within the system, through a "shunt" or side pathway that forms a closed circle with the P700 of photosystem I (Fig. 8-15). In this pathway, electrons pass from the primary acceptor of photosystem to a shunt carrier, cytochrome b6 (also called cytochrome b564), which does not form a part of the regular Z-pathway, From cytochrome b^, electrons pass to cytqchrome f and back again to ground state orbitals in P700, ready for excitation by absorption of additional light quanta. The significance of the| cycle is that at least one ATP molecule is produced each time a pair of electrons travels the circuit around P700. The net result is direct conversion of light to chemical energy in the form of ATP, not necessarily linked to reduction of NADP.
Quantum Requirements of the Z-PathwayThe minimum quanta required to reduce NADP and liberate a molecule of oxygen can be predicted from the pattern of electron flow ! through the Z-pathway. According to Reaction 8-9, splitting one molecule of water liberates V2O2 and transfers two electrons to the Z-pathwaiy. Production of an O2 molecule therefore requires Reaction 8-9 to be doubled:
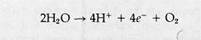
This means that for each O2 molecule generated, four electrons must flow through the Z-pathway to reach two NADP molecules. Each of these electrons will absorb one quantum of light energy in photosystem II and a second in photosystem I, for a total of two quanta per electron. This gives an expected minimum total of eight quanta absorbed for each molecule of O2 evolved in photosynthesis.
ATP Synthesis in the Z-Pathway: Photophosphorylation
D. I. Arnon and his colleagues were the first to demonstrate, I in 1954, that movement of electrons through the Z-pathway I is accompanied by synthesis of ATP. These investigators I
supplied isolated spinach chloroplasts with ADP and inorganic phosphate and detected ATP synthesis in the light. Since the quantity of ATP synthesized was correlated with the amount of electron flow in response to the light, Arnon and his coworkers proposed that ATP synthesis in the isolated chloroplasts was coupled to electron transport.- This light-driven ATP synthesis was termed photophosphorylation.
The mechanism coupling ATP synthesis to electron transport in chloroplasts (reviewed in Jagendorf, 1975) has been most successfully explained by Mitchell's chemiosmotic hypothesis (it might be useful at this point to reread pp. 155-159), which is supported by the following major lines of evidence:
1. Photophosphorylation depends on the presence" of intact,
closed membranous vesicles.
2. Electron transport through photosystems II and I or cyclic
transport around photosystem I results in the accumulation of
H+ ions inside isolated thylakoids or inside vesicles derived
from thylakoids, as predicted by the chemiosmotic hypothesis.
3. Destruction of the H+ gradient by agents that increase per
meability of thylakoid membranes to H+ ions stops ATP
synthesis.
4. Imposition of an artificial H+ gradient across closed
thylakoid membranes leads to a burst of ATP synthesis.
The supporting evidence mentioned in 4, stemming from experiments conducted by A. T. Jagendorf and E. Uribe (1966), is among the strongest in favor of the chemiosmotic hypothesis. Jagendorf and Uribe lowered the internal pH of isolated chloroplasts by placing them in a solution containing acids (at pH 4) that could penetrate the chloroplast membranes. Subsequent transfer to a medium at pH 8 established an H+ gradient that led to an immediate burst of ATP synthesis. The reverse experiment has also been carried out. In 1970, C. Carmeli supplied isolated chloroplast thylakoids with an excess of ATP. Under these conditions, the chloroplast ATPase was forced to run in reverse, hydrolyzing ATP to ADP and phosphate. ATP breakdown in this case led to a buildup of an H+ gradient inside the thylakoids, as expected from an ATPase enzyme running in reverse in accordance with the Mitchell mechanism.
Thus, all evidence indicates that photophosphorylation in chloroplasts and oxidative phosphorylation in mitochondria occur by the same mechanism: Electron transport establishes an H+ gradient, which in turn provides the driving force for ATP synthesis.
The similarities between the two systems extend to the enzyme complex synthesizing ATP. E. Racker and his colleagues (reviewed in Racker, 1976) were able to isolate an ATPase complex from thylakoid membranes. The isolated complex, now called CF^, closely resembles the F! -ATPase
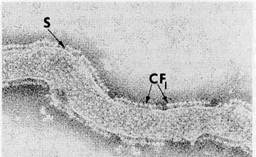
Figure 8-16Isolated, negatively stained thylakoid membranes of spinach chloroplasts showing the CF, ATPase "lollipops." S, stalk, x 100,000. Courtesy of M. P. Garber, from J. Cell Biol. 63: 24(1974), by copyright permission of the Rockefeller University Press.
complex from mitochondria. Removal of CF! from illuminated thylakoid membranes allows electron transport to run without ATP synthesis. Replacement of the CF! complex restores ATP synthesis and reestablishes close coupling between light-driven electron transport and phosphorylation. Removal and restoration of CFi and of ATP-synthesizing capacity is correlated with the disappearance and reappearance of stalked particles on the thylakoid surfaces, as in mitochondrial systems (see Fig. 8-16 and Howell and Moudrianakis, 1967). The particles, which extend from the thylakoid surfaces into the stroma after negative staining, closely resemble the mitochondrial Ft complex in structure and dimensions.
| <== предыдущая лекция | | | следующая лекция ==> |
| The Light and Dark Reactions | | | Organization of the Components of Photosystems I and |