А.П. Анисимов
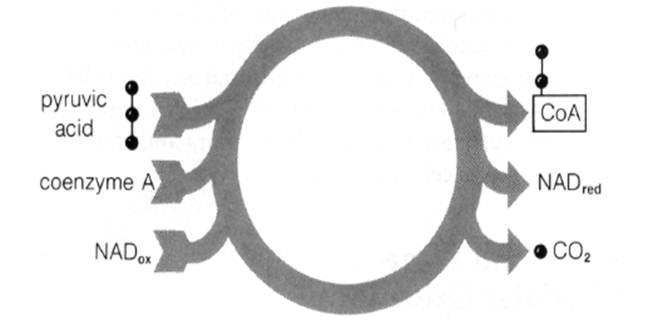
After entering mitochondria, pyruvic acid is shortened to a two-carbon segment in a cyclic series of reactions. These reactions remove two electrons, two hydrogens, and one carbon (as CO2) from the pyruvic acid chain. The two-carbon segment produced by the oxidation is an acetyl ( —CH3CO~) group:
Fig.7-7 Coenzyme A, a carrier of acetyl (— CH3CO ~) groups.
Fig.7-8 The overall reaction of pyruvic acid oxidation.
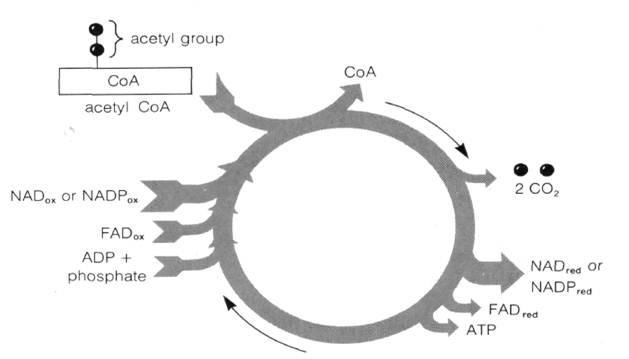
The overall reaction cycle in pyruvic acid oxidation (Fig. 7-8) thus yields as net products acetyl coenzyme A, reduced NAD, and CO2: Fig.7-10 An overview of the Krebs cycle. Fig. 7-11 The reactions and enzymes of the Krebs cycle.
Fig. 7-12 NADP (nicotinamide-adenine dinucleotide phosphate), an alternate electron acceptor (for reaction 3 in Fig. 7-11) of the Krebs cycle.
Fig.7-13 FAD (flavin adenine dinucleotide), an electron acceptor linked to succinic acid dehydrogenase in the cristae membranes of mitochondria. Fig. 7-14 A summary of the Krebs cycle reactions.
Figure 7-15 FMN (flavin mononucleotide). FMN and FAD are the prosthetic groups of the flavoprotein carriers of the electron transport system. The riboflavin group in reduced FMN and FAD binds two hydrogens in the shaded positions to form FMNH2 or FADH2. Figure 7-16 The prosthetic group of cytochrome c, an iron porphyrin group. The iron in the center of the ring, by changing alternately from the ferrous (Fe2+) to the ferric (Fe3+) form, acts as an electron carrier. Similar porphyrin rings occur as central structures in hemoglobin, chlorophyll, and other molecules of biological importance (compare with Fig. 8-8).
Fig.7-17 Coenzyme Q, the only carrier of the electron transport system not linked to a protein. The molecule may exist in the fully oxidized quinone or Q state (a), the partially reduced semiquinone or QH state (b), or the fully reduced hydroquinone or QH2 state (c). The points where hydrogens attach in these reductions are shaded
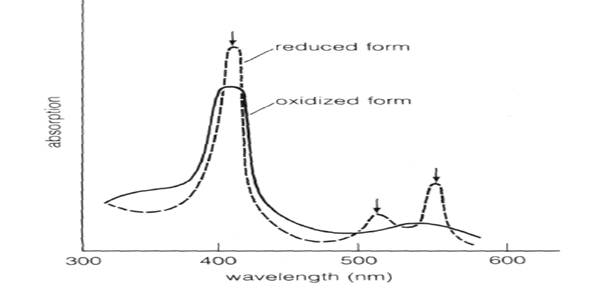
Fig.7-18 The absorption spectra for the oxidized and reduced forms of cytochrome c in solution at pH 7. The oxidized form has one major absorption peak; the reduced form has three (arrows).
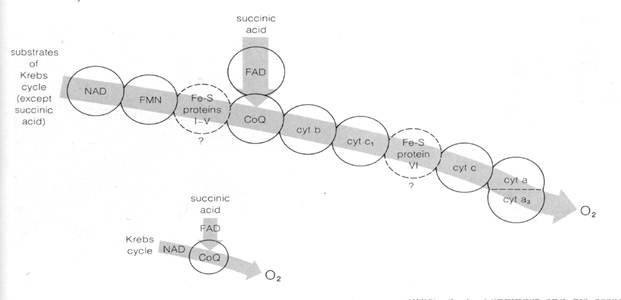
Fig.7-19 A possible sequence for the carriers of the electron transport system of mitochondria). NAD, FMN, FAD, and coenzyme Q are hydrogen-electron carriers. The iron-sulfur proteins (Fe-S) and cytochromes carry only electrons.
Fig. 7-20 The three sites of ATP synthesis in the electron transport chain. Added to the total from Equation 7-13, the overall oxidation of glucose yields 38 molecules of ATP:
Figure 7-23 Mitchell's version of the electron transport chain showing how electron transport sets up a gradient in H+ ions across the mitochondrial membranes.
inside (matrix) 2H+ 1/2O2
Fig.7-24 Mitchell's hypothesis for the mechanism synthesizing ATP in response to a gradient in H+ ions.
Fig. 7-27 The major biochemical pathways oxidizing carbohydrates, fats, and proteins. Coenzyme A, the acetyl carrier, forms a central channel funneling the products of many oxidative pathways into the Krebs cycle.
Fig.7-28 The electron transport system of the bacterium E. coli. The electron carriers are embedded in the plasma membrane in bacteria.
А.П. Анисимов
|