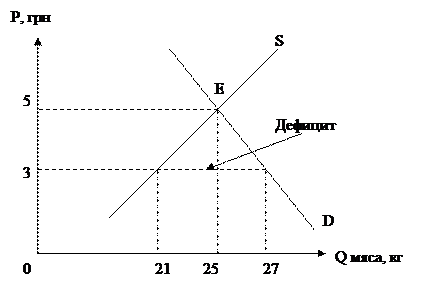
Контрольная работа № 2. Фармацевтического факультета заочного отделения
По английскому языку Для студентов I курса Фармацевтического факультета заочного отделения ВАРИАНТ II
Задание 1: Прочитайте и письменно переведите текст на русский язык. Phosphorus
The element phosphorus is located below nitrogen in Group V of the Periodic Table. It is to be expected that these two elements resemble each other in many respects but there are many points in which they differ radically. The most distinctive feature between these two elements is that nitrogen is quite inactive under ordinary conditions, while phosphorus reacts readily both with metals and non-metals. They differ also in that nitrogen is gas at an ordinary temperature, where as phosphorus is a solid. It is necessary to distinguish white phosphorus and red one. White phosphorus is one of the most igniting substances. It ignites and burns generating much heat. When heating out of contact with air, it melts readily, turning into a colourless liquid. Phosphorus dissolves in many solvents, the best solvent is carbon disulphide, one part of which dissolves nine parts of phosphorus. One has to keep in mind that the most important property of white phosphorus is its activity to oxygen. It is necessary to store white phosphorus under water. Never handle phosphorus with bare hands as heat of the body is sufficient to ignite it. In the soil and in minerals it is found in the form of phosphoric acid salts. The chief minerals containing this salt are apatites and phosphorites.
Пояснение к тексту: to keep in mind - иметь ввиду
Задание 2: Перепишите предложения, подчеркните в каждом инфинитив и укажите его функцию, т.е. определите, будет ли инфинитив подлежащим, обстоятельством, определением или частью составного именного сказуемого. Предложения переведите. 1. The first point to be noted is to keep medicine out of the reach of children. 2. To improve absorption the drug is taken between meals, 3. It is necessary to avoid alcohol if you are taking the medicine. 4. To be careful with a dose to be injected is important.
Задание 3: Перепишите предложения, подчеркните Participle I, укажите его функцию, т. е. определите, будет ли оно определением, обстоятельством или составной частью сказуемого. 1. l. When prescribing the drug the doctor takes into account the possible complications. 2. The medicine possessing a wide spectrum of activity is used more often. 3. If you are suffering from some other illness inform your doctor.
Задание 4: Перепишите предложения; подчеркните глагол-сказуемое и определите его видовременную форму и залог; напишите форму инфинитива. Предложения переведите. 1. The patient is taking this medicine now. 2. When we came into the laboratory they were finishing their experiment. 3. The problem of new antibiotics is being discussed by many scientists both in our country and abroad. 4. The pharmacist will be preparing medicine at 12 o'clock tomorrow. 5. He has disorders in the digestive system, now he is taking tablets during the meal - time.
Задание 5: Перепишите предложения, подчеркните глагол to do и определите, является ли он смысловым, вспомогательным или словом заместителем. Предложения переведите. 1. Do you know the indications of this remedy? 2. My friend does his work with great pleasure. 3. This drug acts in the same way as “Desfcral” does.
Задание 6: Выпишите предложения, где конструкции с глаголами to have, to be выражают долженствование, предложения переведите. 1. If you keep the recommended dose, no serious side effects are to be expected. 2. The substance was heated and evaporated. 3. Many drugs have more than one action. 4. When using these drugs you have not to combine them. 5. 5.The phenomenon of combustion is to be explained by the chemical combination of the burning substance with oxygen of the air.
Задание 7: Перепишите предложения, подчеркните в каждом придаточное предложение; подчинительный союз или союзное слово обведите кружком; при бессоюзном подчинении укажите на место, где мог бы находиться опущенный союз. Предложения переведите. 1. Experiments show us that there is very little attraction between the molecules of any gas. 2. The elements of water is composed of are hydrogen and oxygen. 3. We know the solubility of this substance is very low. 4. Any element that can exhibit different natural forms possesses allotropic forms.
|