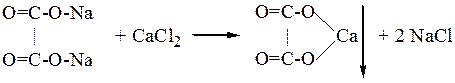Methodical guidance for laboratory work №10
Experiment №1 Oxidation of aldehydes by oxide of silver Pour in clean test-tube 5-6 drops of ammonium solution of silver and add 2-3 drops of 10% solution of acetaldehydes (ethanal) or formaldehyde and hest by hand. Formatted mirror p.p.t. of silver. 2 Ag(NH3)2OH + 3H2O ® Ag2O¯ + 4 NH4OH
Make conclusion. Experiment №2. Oxidation of aldehydes by hydrate of oxide cupper. Pour in clean t-t 5-6 drops of 10 % solution acetaldehyde (ethanal) formaldehyde and 2-3 drops of 10 % of NaOH, add drop by drops of sulfuric cupper till formation muddy solution. And heat easily. At first formated yellow p.p.t. of hydrate of oxide cupper, then red p.p.t. of oxide cupper
Blue p.p.t
| | | | yellow p.p.t O O
red p.p.t Experiment №3 Disproportion of formaldehydes in water solution Pour in clean t-t 2-3 drops of 40 % solution of formalyne and add 1drop of 0.2 % of indicator of metyl red. Red solution show aciditic medium. Make conclusion. Experiment №4 Receiving of vinegarisoamilic ether Pour in clean t-t 10-12 drops of conc. Vinegar acid. isoamilic sprite and 5-6 drop of conc.H2SO4(cat.) Put t-t on the water-box at 50-60oC. Then solution pour in t-t with 1 ml cold water. Formatted vingarisoamilic ether CH3-C-OH+C5H11OHà CH3-C-OC5H11+H2O | | | | O O Make conclusion. Experiment №5. Opening of calcium of sorrelic acid Pour in clean t-t 10-12 drops of sodium salt of sorrelic acid and add 2-3 drops of 2% of CaCl2 till formation of p.p.t calcium salt of sorrelic acid. And add in t-t 5-6 drops of 10 % CH3COOH. p.p.t not dissolved. Then add 5-6 drops of 10 % HCl. p.p.t is dissolved red. Make conclusion.
6. Literature: 1. J. M. Sehgal. Modern Chemistry, class XI, Delhi, 1989 - 15 items. 2. Jean B. Umland & John M. Bellama, General Chemistry. Houston. USA. 1996 - 1 item. 3. Darrel D. Ebbing, General Chemistry, Wayne University, USA. 1990 - 3 items. 4. Karen C. Timberlake, Chemistry - An Introduction to General. Organic and Biochemistry, Los Angeles, USA, 1991-1 item. 7.Controle: № 1. What compounds are got at interaction propanal with methyl by alcohol? Write the equation of reactions. №2. Write the reaction an aldol condensation. Why this reaction characteristic only aldehydes, having hydrogen atom in a-carbon? №3. Write the equation of reactions of the reception ethyl acetate from ethyl alcohol and acetic acid. Explain the mechanism of reaction. №4. Complex ester of phenylsalylilate фенилсалициалат is used inside under intestine disease and hydrolysis in basic medium of the bowels. Write the equation an hydrolytic fissions of phenylsalylilate. №5. Write the reactions 1) decarboxylation of malonic acid 2) formation of anhydride of malonic acids.
|

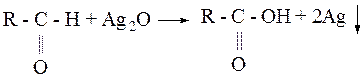
 CuSO4+2NaOHà Cu(OH)2+Na2SO4
CuSO4+2NaOHà Cu(OH)2+Na2SO4 R-C-H+Cu(OH)2àR-C-OH+2CuOH +2CuOH +H2O
R-C-H+Cu(OH)2àR-C-OH+2CuOH +2CuOH +H2O