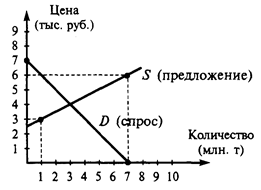
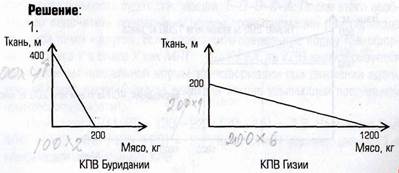
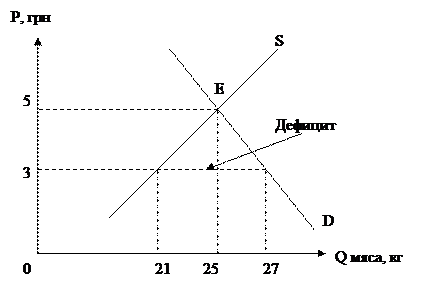
Language Practice Translate into English
ЧТО РЕШИЛ СУД В соответствии со ст. 1 Закона Украины "О режиме иностранного инвестирования" №93/96-ВР от 19.03.96 г., иностранные инвестиции -ценности, которые вкладываются иностранными инвесторами в объекты инвестиционной деятельности в соответствии с законодательством Украины в целях получения прибыли или достижения социального эффекта. Предприятие с иностранными инвестициями - это предприятие (организация) какой-либо организационно-правовой формы, созданное в соотвествии с законодательством Украины, иностранная инвестиция в уставном фонде которого, при его наличии, составляет не менее 10%. Предприятие приобретает статус предприятия с иностранными инвестициями со дня зачисления иностранной инвестиции на его баланс. Статьей 13 этого Закона предусмотрена обязательность государственной регистрации иностранной инвестиции, а ст. 16 этого Закона предусмотрено, что учредительные документы предприятий с иностранными инвестициями должны содержать сведения о государственной принадлежности учредителей. В соотвествии с нормативными актами, регулирующими деятельность органов таможенной службы, к их компетенции не отнесено право определять
млн присваивать субъекту внешнеэкономической предприятия с иностранными инвестициями. По материалам еженедельника "Бизнес "; Supplementary texts Text A Patient capital India has such people in industrial quantities, and contract-research organisations (CROS), which undertake clinical trials and other services for pharmaceutical companies, are beginning to notice. Quintiles Transnational, a big CRO based in North Carolina, is one pioneer. Its Indian joint venture began three years ago and now has a staff of 40 and a score of customers, mainly American and European pharmaceutical and biotech firms. Co-vance, a CRO from New Jersey, has started clinical trials in India and may expand its operations. Indian companies have also spotted an opportunity. Nicholas Piramal, a pharmaceutical company in Mumbai, recently hired a chief executive for a new clinical-trials business that it hopes will have revenues of 100m-120m Indian rupees ($2.3m-2.8m) within three years. Max India, which among other activities makes bulk phar-maceuticals, has signed a deal with an affiliate of Harvard Medical School to conduct clinical trials in India and in other developing countries. The potential return on investment in India's infrastructure for conducting clinical trials "is tremendous," says Renu Gupta, Covance's vice-president of global safety and medical therapeutics. The advantages look so obvious it seems a wonder that more companies have not entered the market sooner. Discovering and developing a new drug is expensive: the industry claims to spend as much as $500m to bring a new molecule to market. A big chunk, perhaps a third of the total, goes towards clinical trials. Much of that is spent just before seeking regulatory approval on "phase-three" trials that use lots of human subjects. A few years ago, the biggest drug-consuming countries, the United States, Japan and the European Union, agreed on common standards for running clinical trials. In principle, America's Food and Drug Administration should now accept data from a trial anywhere in the world that meets those standards. Clinical trials in India ought to be cheaper and faster than those in developed markets. CROS can hire researchers, nurses and computer staff at less than a third of western wages. India's billion potential guinea-pigs suffer not just from tropical and poor-country diseases such as malaria and tuberculosis, but increasingly from ailments such as cancer, heart disease and aids, which trouble rich countries. Though Indians are spread out and mainly live in rural areas, people with serious ailments flock to advanced hospitals in cities, which makes them accessible. From the Economist Text В A penchant for patents Hungary's pharmaceuticals industry is not about to ditch its penchant for patents, pointing to a reputation for research that stretches back to Nobel Laureate Albert S/cnt- Gyorgyi, the discoverer of Vitamin С But while the country still has some clever researchers, its pharma companies are too small to exploit their discoveries commercially. Human, a small pharmaceutical company, is the latest example of Hungarian research flair. It recently took out a 20-vear global patent on its new anti-cancer compound, TaxalB. Certain that it's on to a winner, Human has poured 5420,000 into developing the drug, a tenth of the cost in the US, analysts reckon. The company expects millions of dollars in return, if it can find a foreign company to market and produce the vaccine world-wide. Fortunately, multinationals are queuing up to buy Hungarian drugs at the moment - and the companies that discover them. The country's two largest drug developers have both brought in US investors over the past two years. The Institute for Drug Research was bought by New York-based Paramount Capital Investments last year, and works closely with US pharma giant Eli Lilly to test its drugs. Biorex Research and Development sold a minority stake to America's Abbott Laboratories, which is helping to develop its promising liimoclomol diabetes drug in exchange for 10% of the royalties from sales. So while Hungarians are discovering new drugs, they need foreign help to bring them to market. And the signs are that if such drugs enter production, they will be produced by foreign companies as well. Low costs and Hungary's reputation for innovation explain multinational drug-makers' interest in the country. But the sad side is that there are virtually no Hungarian companies left capable of producing original drugs themselves. From Business Central Europe Comprehension tasks 1. Summarize the main points from the articles in your own words. 2. Why do investments into pharmaceuticals industry yield high returns? 3. What other areas look attractive to investors? 4. The investment policy is based upon the recognition of two conflicting 5. Choose a company in your country. Find out as much as you can about it
|