The Photoelectric Effect
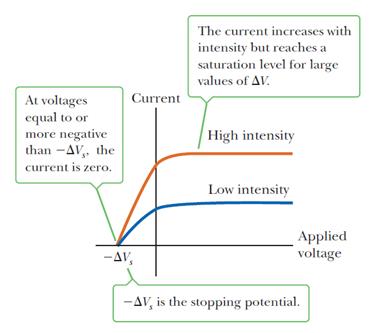
In a Photocell, a photon is coming in with Energy Ephoton = hf = pc If the photon has enough energy, it will “kick out” or emit electrons from atoms in metal cathode. The Photocell is then connected to a circuit, and the freed electrons travel in the circuit and create a photocurrent. We can find the kinetic energy of these electrons by supplying a very small voltage difference between the anode and cathode, opposing the photocurrent. The smallest voltage that stops the photocurrent is Vs.
• The current arises from photoelectrons emitted from the negative plate and collected at the positive plate; • For each metal, there is a threshold frequency below which no electrons are emitted; • The number of electrons emitted is proportional to intensity of radiation; • Emitted electrons have KE up to a maximum value, which depends on the frequency of radiation (unexplained by classical physics). • When the applied potential difference is equal to or more negative than -ΔVs, the stopping potential, no electrons reach the anode, and the current is zero. • The stopping potential is independent of the radiation intensity. • Some electrons are ejected with slower speeds. The most energetic electrons have KEMAX = e∆VS
|