1) 2С2Н5ОН +2Na → 2C2H5 – O – Na + H2
2) C2H5ONa + H2O → C2H5OH + 2NaOH
3) C2H5OH + HCl → C2H5Cl + H2O
4) C2H5OH + PCl5 → C2H5Cl + POCl3 + HCl
H2SO4
 5) C2H5OH CH2 ═ CH2 + H2O
5) C2H5OH CH2 ═ CH2 + H2O
t 1700
H2SO4
 6) 2C2H5OH t 1400 C2H5 – O – C2H5 + 2H2O
6) 2C2H5OH t 1400 C2H5 – O – C2H5 + 2H2O

 O H+ O
O H+ O

 7) R – C + HO – C2H5 → R – C + H2O
7) R – C + HO – C2H5 → R – C + H2O
OH t0 O – C2H5
8) C2H5OH + 3O2 → 2CO2 + 3H2O
Окисление первичных спиртов
 t0 O
t0 O
 9) C2H5OH + CuO → CH3 – C + Cu + H2O (мягкое)
9) C2H5OH + CuO → CH3 – C + Cu + H2O (мягкое)
H
 Na2Cr2O7 O
Na2Cr2O7 O

 10) С2H5OH CH3 - C (жёсткое)
10) С2H5OH CH3 - C (жёсткое)
Н+, t0 OH
11)Окисление вторичных спиртов
K2Cr2O7
 СН3 – СH – CH3 CH3 – C – CH3
СН3 – СH – CH3 CH3 – C – CH3
│ H+ ║
OH O
t0
12) CH3 – CH2 – OH + Cl2 → CH3 – CH – OH + HCl
│
Cl
 13) Дегидрирование Cu, t0 O
13) Дегидрирование Cu, t0 O

 СН3 – CH2 – OH CH3 – C + H2
СН3 – CH2 – OH CH3 – C + H2
H
Получение:
t0, кат.
 1) СH4 + H2O СО + 3Н2
1) СH4 + H2O СО + 3Н2
Cr2C3 + 2nO
 СО + Н2 t 0, P CH3OH + H2O
СО + Н2 t 0, P CH3OH + H2O
брожжение
 2) C6H12O6 2С2Н5OH + 2СО2
2) C6H12O6 2С2Н5OH + 2СО2
3) С2H5 – O – C2H5 + HI → C2H5I + C2H5OH

 O O
O O

 4) CH3 – C + NaOH → CH3 – C + CH3OH
4) CH3 – C + NaOH → CH3 – C + CH3OH
O – CH3 O – Na
H+
5) CH2 ═ CH2 + H2O → C2H5OH
 6) C2H5Cl + NaOH водн. C2H5OH + HCl
6) C2H5Cl + NaOH водн. C2H5OH + HCl
LiAlH4
 7) CH3COOH CH3 – CH2 – OH
7) CH3COOH CH3 – CH2 – OH
LiAlH4
 8) CH3COH CH3 – CH2 – OH
8) CH3COH CH3 – CH2 – OH
LiAlH4
 9) CH3 – C – CH3 CH3 – CH – CH3
9) CH3 – C – CH3 CH3 – CH – CH3
║ │
O OH
Альдегиды
Химические свойства
 О
О

 1) СН3 – С + НСN → CH3 – CH - OH
1) СН3 – С + НСN → CH3 – CH - OH
Н ;
CN
 O
O

 2) CH3 – C + HOH → CH3 – CH - OH
2) CH3 – C + HOH → CH3 – CH - OH
H
OH
 O
O

 3) CH3 – C + CH3OH → CH3 – CH– O – CH3
3) CH3 – C + CH3OH → CH3 – CH– O – CH3
H
OH
 O t0, Pt
O t0, Pt

 4) CH3 - C + H2 CH3 – CH2OH
4) CH3 - C + H2 CH3 – CH2OH
H
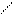
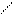 O t0 O
O t0 O

 5) CH3 - C + Ag2O → CH3 – C + 2Ag↓
5) CH3 - C + Ag2O → CH3 – C + 2Ag↓
H OH

 O t0 O
O t0 O

 6) 2CH3 – C + 2Cu(OH)2 → 2CH3 – C + Cu2O + H2O
6) 2CH3 – C + 2Cu(OH)2 → 2CH3 – C + Cu2O + H2O
H OH
CH2
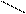
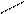


 O O O
O O O

 7) 3HC
7) 3HC

 H CH2 CH2
H CH2 CH2
O
 CH3 O
CH3 O



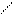

 O CH − CH – CH3
O CH − CH – CH3

 8) 3CH3 – C
8) 3CH3 – C
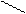
 H O O
H O O
CH – CH3



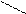





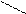
 O t, кат.
O t, кат.



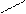


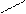
 9) nHC + n + nH2O
9) nHC + n + nH2O


 H -CH2 CH2 -
H -CH2 CH2 -
OH OH n
 O
O
 10) CH3 – C + PCl5 → CH3 – CH – Cl2 + POCl3
10) CH3 – C + PCl5 → CH3 – CH – Cl2 + POCl3
H

 O hν O
O hν O


 11) CH3 – C + Cl2 CH2 – C + HCl
11) CH3 – C + Cl2 CH2 – C + HCl
 H H
H H
Cl


 O O O
O O O



 12) CH3 - C + CH3 – C CH3 – CH – CH2 – C
12) CH3 - C + CH3 – C CH3 – CH – CH2 – C
H H │ H
 O OH
O OH
 13) 2HC + NaOH → CH3OH + CH3COONa
13) 2HC + NaOH → CH3OH + CH3COONa
H
Получение:  Hg2+ O
Hg2+ O

 1) СH ≡ CH + H2O CH3 - C
1) СH ≡ CH + H2O CH3 - C
H
Hg2+
 2) CH3 – C ≡ CH + H2O CH3 – C – CH3
2) CH3 – C ≡ CH + H2O CH3 – C – CH3
║
O
O 


 3) CH3 – CHCl2 + NaOH → CH3 – CH – OH → CH3 – C + NaCl + H2O
3) CH3 – CHCl2 + NaOH → CH3 – CH – OH → CH3 – C + NaCl + H2O
H
OH
4) CH3– CCl2–CH3 +NaOH→ CH3–C(OH)2 –CH3→ CH3 – C –CH3 + NaCl + H2O
║
 t0 O O
t0 O O
 5) CH3 – CH2OH + CuO → CH3 –C + Cu + H2O
5) CH3 – CH2OH + CuO → CH3 –C + Cu + H2O
H
KMnO4
 6) CH3 – CH – CH3 CH3 – C – CH3
6) CH3 – CH – CH3 CH3 – C – CH3
│ H+, t0 ║
OH O
Ca(OH)2
 7) (CH3COO)Ca t0 CH3 – C – CH3 + CaCO3
7) (CH3COO)Ca t0 CH3 – C – CH3 + CaCO3
║
O
Получение формальдегида в промышленности
O2, Ag/ t0
 8) CH3OH HCOH
8) CH3OH HCOH
Получение ацетальдегида в промышленности
O2, Ag /t0
 9) СH3 – CH2OH CH3 – COH
9) СH3 – CH2OH CH3 – COH
Дегидрирование спиртов:
Cu, t0
 10) СH3 – CH2OH CH3 – COH + H2
10) СH3 – CH2OH CH3 – COH + H2
 Pd, O2 O
Pd, O2 O

 11) CH3 – CH = CH – CH3 2CH3 – C H
11) CH3 – CH = CH – CH3 2CH3 – C H

 5) C2H5OH CH2 ═ CH2 + H2O
5) C2H5OH CH2 ═ CH2 + H2O 6) 2C2H5OH t 1400 C2H5 – O – C2H5 + 2H2O
6) 2C2H5OH t 1400 C2H5 – O – C2H5 + 2H2O

 t0 O
t0 O 9) C2H5OH + CuO → CH3 – C + Cu + H2O (мягкое)
9) C2H5OH + CuO → CH3 – C + Cu + H2O (мягкое) Na2Cr2O7 O
Na2Cr2O7 O
 10) С2H5OH CH3 - C (жёсткое)
10) С2H5OH CH3 - C (жёсткое) СН3 – СH – CH3 CH3 – C – CH3
СН3 – СH – CH3 CH3 – C – CH3 13) Дегидрирование Cu, t0 O
13) Дегидрирование Cu, t0 O СН3 – CH2 – OH CH3 – C + H2
СН3 – CH2 – OH CH3 – C + H2 1) СH4 + H2O СО + 3Н2
1) СH4 + H2O СО + 3Н2 СО + Н2 t 0, P CH3OH + H2O
СО + Н2 t 0, P CH3OH + H2O
 6) C2H5Cl + NaOH водн. C2H5OH + HCl
6) C2H5Cl + NaOH водн. C2H5OH + HCl 7) CH3COOH CH3 – CH2 – OH
7) CH3COOH CH3 – CH2 – OH 8) CH3COH CH3 – CH2 – OH
8) CH3COH CH3 – CH2 – OH 9) CH3 – C – CH3 CH3 – CH – CH3
9) CH3 – C – CH3 CH3 – CH – CH3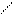 О
О

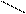
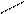



 H CH2 CH2
H CH2 CH2




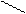
 H O O
H O O


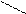


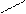


 H -CH2 CH2 -
H -CH2 CH2 -
 H H
H H
 O OH
O OH 2) CH3 – C ≡ CH + H2O CH3 – C – CH3
2) CH3 – C ≡ CH + H2O CH3 – C – CH3
 t0 O O
t0 O O 5) CH3 – CH2OH + CuO → CH3 –C + Cu + H2O
5) CH3 – CH2OH + CuO → CH3 –C + Cu + H2O 6) CH3 – CH – CH3 CH3 – C – CH3
6) CH3 – CH – CH3 CH3 – C – CH3 7) (CH3COO)Ca t0 CH3 – C – CH3 + CaCO3
7) (CH3COO)Ca t0 CH3 – C – CH3 + CaCO3 9) СH3 – CH2OH CH3 – COH
9) СH3 – CH2OH CH3 – COH 10) СH3 – CH2OH CH3 – COH + H2
10) СH3 – CH2OH CH3 – COH + H2