NaOH

 СH3 – CH2 – Cl водн. CH3 – CH2 – OH + NaCl
СH3 – CH2 – Cl водн. CH3 – CH2 – OH + NaCl
NH3 C2H5 – NH2 + HCl
 H2O
H2O


 KCN C2H5C ≡ N C2H5COOH + NH4OH + KCl
KCN C2H5C ≡ N C2H5COOH + NH4OH + KCl
CH3ONa CH3 – CH2 – O – CH3 + NaCl
 O
O

 CH3 – C
CH3 – C


 ONa O
ONa O

 CH3 – C
CH3 – C
O – CH2 – CH3 + NaCl
KOH СH2 = CH2 + KCl + H2O
 спирт
спирт
 Na CH3 – CH2 – CH2 – CH3 + NaCl
Na CH3 – CH2 – CH2 – CH3 + NaCl
KOH
 CH2Cl – CH2Cl спирт CH ≡ CH + KCl + H2O
CH2Cl – CH2Cl спирт CH ≡ CH + KCl + H2O
 t0
t0
CH2Cl – CH2Cl + Zn пыль СH2 = CH2 + ZnCl2
СH2

 Zn
Zn

 CH2Cl – CH2 – CH2Cl пыль H2C CH2 + KCl + H2O
CH2Cl – CH2 – CH2Cl пыль H2C CH2 + KCl + H2O
абсол.
 C2H5 – Cl + Mg эфир С2H5 – Mg – Cl
C2H5 – Cl + Mg эфир С2H5 – Mg – Cl
C2H5MgCl + HCl → C2H6 + MgCl2
 O
O
 C2H5MgCl + H – C + HCl → C2H5 – CH2 – OH + MgCl2
C2H5MgCl + H – C + HCl → C2H5 – CH2 – OH + MgCl2
H первичный спирт
 O
O
 C2H5MgCl + CH3 – C + HCl → C2H5 – CH – CH3 + MgCl2
C2H5MgCl + CH3 – C + HCl → C2H5 – CH – CH3 + MgCl2
 H
H
OH вторичный спирт
CH3

C2H5MgCl + CH3 – C - CH3 + HCl → C2H5 – C – CH3 + MgCl2
 ║
║
O OH третичный спирт
 O
O
 C2H5MgCl + CO2 + HCl → C2H5 – C + MgCl2
C2H5MgCl + CO2 + HCl → C2H5 – C + MgCl2
OH
Алкадиены
H2


 СH2 ═ CH – CH ═ CH2 Ni CH3 – CH ═ CH – CH3
СH2 ═ CH – CH ═ CH2 Ni CH3 – CH ═ CH – CH3
Br2 CH2 – CH ═ CH – CH2


Br Br
HCl
 CH3 – CH ═ CH – CH2 – Cl
CH3 – CH ═ CH – CH2 – Cl
 nCH2 ═ CH – CH – CH2 (- CH2 – CH ═ CH – CH2 -)n
nCH2 ═ CH – CH – CH2 (- CH2 – CH ═ CH – CH2 -)n
Получение:
Al2O3, Cr2O3
 CH3 – CH2 – CH2 – CH3 CH2 ═ CH – CH ═CH2 + 2H2
CH3 – CH2 – CH2 – CH3 CH2 ═ CH – CH ═CH2 + 2H2
t0
ZnO, Al2O3
 2CH3 – CH2 – OH t0 CH2 ═ CH – CH ═ CH2 + 2H2O + H2
2CH3 – CH2 – OH t0 CH2 ═ CH – CH ═ CH2 + 2H2O + H2
MnO, ZnO
 CH3 – CH ═ CH – CH3 CH2 ═ CH – CH ═ CH2 + H2
CH3 – CH ═ CH – CH3 CH2 ═ CH – CH ═ CH2 + H2
t0
Фенол

 OH ONa
OH ONa





 1) 2 + 2Na → 2 + H2↑
1) 2 + 2Na → 2 + H2↑
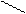


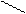

 OH O – Na
OH O – Na




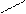


 2) + NaOH → + H2O
2) + NaOH → + H2O

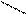





 3) + PCl5 → + POCl3 + HCl
3) + PCl5 → + POCl3 + HCl

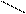

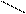


OH Cl
 C2H5
C2H5




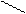
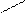 H2SO4
H2SO4









 4) 2 + 2CH2 ═ CH2 +
4) 2 + 2CH2 ═ CH2 +

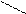


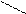

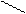


 C2H5
C2H5
OH OH OH
SO3H

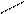
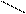
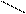
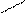
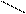
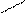










 5) 2 + 2H2SO4(к) → + + H2O
5) 2 + 2H2SO4(к) → + + H2O

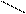


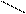
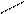

 SO3H
SO3H
OH OH OH
NO2




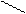



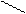

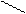

 6) t0
6) t0





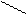 + 3HNO3(к) → + 3H2O
+ 3HNO3(к) → + 3H2O
NO2 NO2
OH OH
 Br
Br

















 7) + 3Br2 → + 3HBr
7) + 3Br2 → + 3HBr
Br Br
OH OH
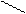
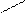
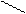
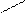 H+
H+










 8) + C2H5Cl + H2O + NaCl
8) + C2H5Cl + H2O + NaCl
ONa O – C2H5










 9) +Zn
9) +Zn

OH





 O
O






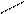
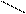

 10) n + nH – C + nH2O
10) n + nH – C + nH2O





 H
H
-H2C CH2 -
OH OH n
Получение:
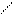


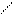

 Cl t0 OH
Cl t0 OH





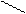

 1) + NaOH → + NaCl
1) + NaOH → + NaCl

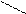


 ONa OH
ONa OH







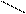
 2) + HCl → + NaCl
2) + HCl → + NaCl






 3) [O] + CH3 – C – CH3
3) [O] + CH3 – C – CH3



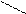

 ║
║
O
CH3 – CH – CH3 OH
Алканы
hν
 1) СH3 – CH3 + Cl2 CH3 – CH2 – Cl + HCl
1) СH3 – CH3 + Cl2 CH3 – CH2 – Cl + HCl
Реакция Коновалова t0, P
 2) CH3 – CH2 - CH3 + HNO3 CH3 – CH – CH3 + H2O
2) CH3 – CH2 - CH3 + HNO3 CH3 – CH – CH3 + H2O

NO2
3) CH4 + 2O2 → CO2 + 2H2O + Q
Ag, O2 окисление
 4) CH3 – CH3 C2H5OH, CH3COOH, CH3COH
4) CH3 – CH3 C2H5OH, CH3COOH, CH3COH
ALCl3
 5) СH3 – CH2 – CH2 – CH3 CH3 – CH – CH3
5) СH3 – CH2 – CH2 – CH3 CH3 – CH – CH3
 t0
t0
CH3
t0, Pt (Al2O3)
 6) CH3 – CH3 CH2 ═ CH2 + H2
6) CH3 – CH3 CH2 ═ CH2 + H2
t0, кат.
 7) C10H22 С5H10 + С5H12
7) C10H22 С5H10 + С5H12
t0
 8) CH4 C + 2H2
8) CH4 C + 2H2
15000C
 9) 2CH4 C2H2 + 3H2
9) 2CH4 C2H2 + 3H2
Получение:
t0, Ni
 1) СH2 ═ CH2 + H2 CH3 – CH3
1) СH2 ═ CH2 + H2 CH3 – CH3
2) Al4C3 + 12H2O → 4Al(OH)3 + 3CH4
Реакция Вюрца
3) 2CH3Cl + 2Na → CH3 – CH3 + 2NaCl
сплавл.
 4) CH3 – COONa + NaOH CH4 + Na2CO3
4) CH3 – COONa + NaOH CH4 + Na2CO3
Синтез Кольбе электролиз
 5) 2CH3 – COONa + 2H2O СH3 – CH3 + 2NaOH + H2 + 2CO2
5) 2CH3 – COONa + 2H2O СH3 – CH3 + 2NaOH + H2 + 2CO2
6) СH3I + HI → CH4 + I2
7) CH3I + H2 → CH4 + HI


 СH3 – CH2 – Cl водн. CH3 – CH2 – OH + NaCl
СH3 – CH2 – Cl водн. CH3 – CH2 – OH + NaCl H2O
H2O

 O
O
 CH3 – C
CH3 – C
 ONa O
ONa O
 спирт
спирт Na CH3 – CH2 – CH2 – CH3 + NaCl
Na CH3 – CH2 – CH2 – CH3 + NaCl CH2Cl – CH2Cl спирт CH ≡ CH + KCl + H2O
CH2Cl – CH2Cl спирт CH ≡ CH + KCl + H2O t0
t0
 Zn
Zn
 CH2Cl – CH2 – CH2Cl пыль H2C CH2 + KCl + H2O
CH2Cl – CH2 – CH2Cl пыль H2C CH2 + KCl + H2O C2H5 – Cl + Mg эфир С2H5 – Mg – Cl
C2H5 – Cl + Mg эфир С2H5 – Mg – Cl O
O O
O C2H5MgCl + CH3 – C + HCl → C2H5 – CH – CH3 + MgCl2
C2H5MgCl + CH3 – C + HCl → C2H5 – CH – CH3 + MgCl2 H
H  ║
║  O
O C2H5MgCl + CO2 + HCl → C2H5 – C + MgCl2
C2H5MgCl + CO2 + HCl → C2H5 – C + MgCl2

 CH3 – CH ═ CH – CH2 – Cl
CH3 – CH ═ CH – CH2 – Cl nCH2 ═ CH – CH – CH2 (- CH2 – CH ═ CH – CH2 -)n
nCH2 ═ CH – CH – CH2 (- CH2 – CH ═ CH – CH2 -)n CH3 – CH2 – CH2 – CH3 CH2 ═ CH – CH ═CH2 + 2H2
CH3 – CH2 – CH2 – CH3 CH2 ═ CH – CH ═CH2 + 2H2 2CH3 – CH2 – OH t0 CH2 ═ CH – CH ═ CH2 + 2H2O + H2
2CH3 – CH2 – OH t0 CH2 ═ CH – CH ═ CH2 + 2H2O + H2 CH3 – CH ═ CH – CH3 CH2 ═ CH – CH ═ CH2 + H2
CH3 – CH ═ CH – CH3 CH2 ═ CH – CH ═ CH2 + H2




















 + 3HNO3(к) → + 3H2O
+ 3HNO3(к) → + 3H2O





 H
H






 6) CH3 – CH3 CH2 ═ CH2 + H2
6) CH3 – CH3 CH2 ═ CH2 + H2 7) C10H22 С5H10 + С5H12
7) C10H22 С5H10 + С5H12 8) CH4 C + 2H2
8) CH4 C + 2H2 1) СH2 ═ CH2 + H2 CH3 – CH3
1) СH2 ═ CH2 + H2 CH3 – CH3 5) 2CH3 – COONa + 2H2O СH3 – CH3 + 2NaOH + H2 + 2CO2
5) 2CH3 – COONa + 2H2O СH3 – CH3 + 2NaOH + H2 + 2CO2