Cathodic Protection
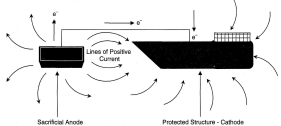
The Electrochemical Theory of Corrosion The electrochemical theory of corrosion states that corrosion proceeds by an anodic or oxidation reaction and a cathodic or reduction reaction. Hydroxyl ions migrate through the water to the anode, here combining with the iron ions to form Fe(OH)2 which combines with dissolved oxygen to form Fe(OH)3 or rust. In this way the anodic area will corrode. To prevent this it would be necessary to make the entire hull cathodic. Therefore, forcing electrons onto the metal will stop its corrosion. This technique is called "Cathodic Protection". The outer surface of a ship's hull is subjected to electro-chemical attack by corrosive currents that flow between areas of the hull, which are at slightly different electric potentials. Dissimilar metals, variations in structural and chemical uniformity in hull plates and welding, differences in paint thickness and quality, water temperature, salinity and aeration combine to cause areas of the hull to become either anodes (positive) or cathodes (negative). The value of protection current must be critically controlled to just prevent corrosion, as beyond this value the increase in the rate of release of hydroxyl ions will cause sponginess and flaking of the anti-fouling paint. Reference electrodes can determine the correct value of protection current. These are either made of zinc or silver attached to the hull below the waterline, but insulated from it.
|