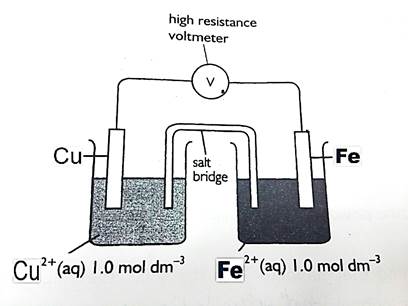
Experiment 4: Constructing an iron/ copper cell. ü Equations: Fe0(s) → Fe2+(aq) + 2e-
ü Equations: Fe0(s) → Fe2+(aq) + 2e- Cu2+ (aq) + 2e- → Cu0(s) Cu2+ (aq) + Fe0(s) → Fe 2+ (aq) + Cu0(s) ü Cell diagram:
ü The polarity of the electrodes:
Cu2+ │Cu0 ││Fe0 │Fe2+
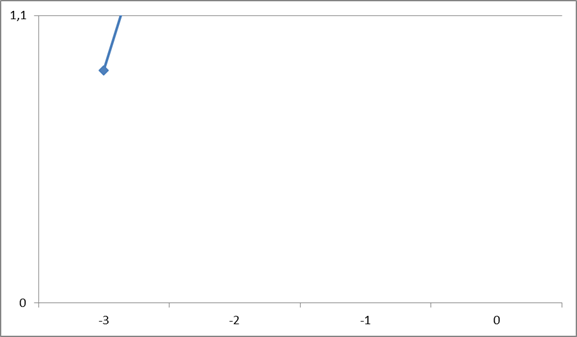
Table 1: Results of electrochemical data of cells.
V. Discussion
VI. Conclusions VII. References
|