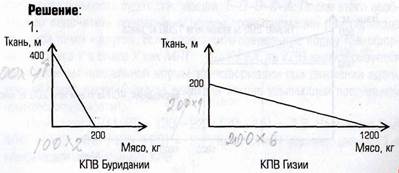
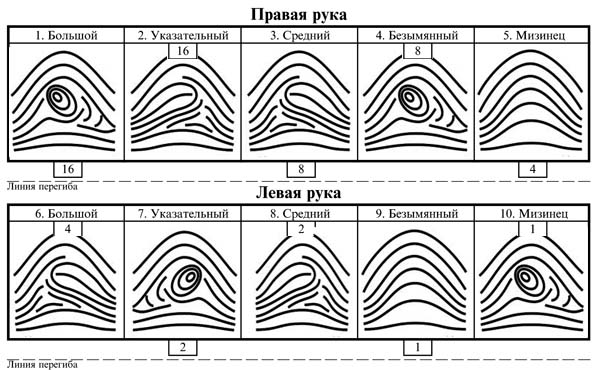
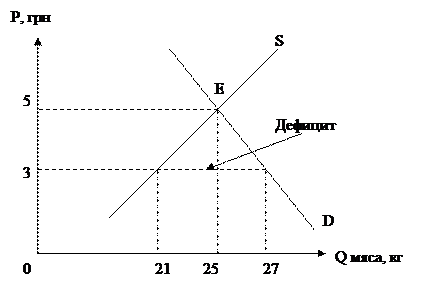
The current-induced local oxidation (CILO) process
The CILO process is based on thin Ti films as illustrated in the sketch and the AFM picture above. Subsequently, oxide notches are via AFM oxidation such that a narrow metallic gap is formed.
Upon applying a constant bias voltage across the gap under ambient conditions (in air), the measured current starts to decrease rapidly as a function of time, stabilizing seconds later at a two orders of magnitude lower value. In the meantime, the gap has been closed by a current-induced nanometer-scale oxide barrier. After the barrier formation the current-voltage curve became strongly nonlinear (red, top right), while it was linear before (blue). The CILO process occurs at current densities of 107 A/cm2 and is triggered by current-induced atomic rearrangements and local heating.
The conductance-time curve provides a measure for the oxidation rate during the CILO process. At the final stages of the barrier formation, when only nanometer-scale channels within the gap remain metallic, the oxidation rate decreases drastically and the conductance drops in steps of about 2e2/h (black, top left and right). These steps give evidence of conductance quantization as a consequence of ballistic transport. The reduced oxidation rate indicates a superior stability of such metallic nanowires against current-induced forces compared with the bulk metal. Thus, the CILO process provides a striking example, where quantum transport influences a chemical reaction.
|