Overall assessment of the knowledge 1 страница
A teacher analyses competencies: knowledge, self-education on the theme, analyses mistakes that were done by students at the passing of tests. At the end of the lesson corresponding points are put.
Major: General medicine Module of: Medical biophysics and biostatistics
CONTROL AND MEASURING MEANS FOR THE FINAL ASSESSMENT OF KNOWLEDGE AND SKILLS ON “MEDICAL BIOPHYSICS” DISCIPLINE
Course: 1 Discipline: Medical biophysics
Discussed on session of the module Protocol № from year Approved Head of the module, professor________ Nurmaganbetova М.О.
CONTROL AND MEASURING MEANS FOR THE FINAL ASSESSMENT OF KNOWLEDGE AND SKILLS ON “MEDICAL BIOPHYSICS” DISCIPLINE FOR 051101 “GENERAL MEDICINE” MAJOR Multiple choice questions on “Medical biophysics” discipline 1. The base of the structure of a membrane is: 1. double lipid layer; 2. crystalline lattice; 3. water solution; 4. red blood cells rouleaus; 5. taste receptors. 2. Properties of phospholipid molecules that are in the structure of biological membranes: 1. one part is hydrophilic, another one is hydrophobic; 2. one part is proteins, another one is hydrophilic; 3. one part is proteins, another one is hydrophobic; 4. chemically neutral; 5. nonpolar. 3. Properties of the membranes: 1. stable, have electrical insulating properties, flexible; 2. superconductivity, flexible; 3. superfluidity, superconductivity; 4. ability to emit radiation, flexible. 5. ability to ionize, flexible. 4. Lipid bilayer of membrane consists of: 1. nonpolar head and polar tail; 2. monolayer phospholipid; 3. cholesterol; 4. charged photons; 5. polar head and nonpolar tail. 5. Functions of membrane proteins: 1. provide the transport of hydrophilic substances through the membrane; 2. implement the superfluidity; 3. implement the transmission of pulse wave; 4. serve as the source of electromagnetic wave; 5. increase the pressure. 6. Fick’s law for the passive transferring of substances through the membrane: 1. 2. 3. 4. 5. 7. Thickness of the membranes: 1. is about several millimetres; 2. is about several nanometers; 3. is about several decimeters; 4. is about several centimetres; 5. is about several metres. 8. Main functions of biological membranes: 1. mechanical, matrix, barrier; 2. wave, matrix, insulating; 3. insulating, structural, mechanical; 4. structural, wave, mechanical; 5. wave, matrix, structural. 9. According to the fluid-mosaic model biological membrane consists of: 1. bilipid layer; 2. two layers with protein layer between them; 3. two lipid layers that are surrounded from above and below with two protein layers; 4. bilipid layer, proteins and microfilaments; 5. layer of lipids with inclusions of proteins and carbohydrates. 10. Lateral diffusion is the diffusion of: 1. molecules from one lipid layer to another one; 2. molecules through the biological membrane; 3. molecules in membrane within one layer; 4. protein molecules from one lipid layer to another one; 5. ions through the bilayer membrane. 11. Transition of molecules from one lipid layer to another one is called 1. “flip-flop” transition; 2. facilitated diffusion; 3. active transport; 4. lateral diffusion; 5. passive transport. 12. Liposomes are: 1. monomolecular layers on the intefase between the hydrophobic and hydrophilic phases; 2. flat bilayer lipid membranes; 3. bilipid enclosed structures; 4. layers of lipids and proteins that are applied on the water surface; 5. the same as micelles. 13. Lipids in biological membranes are in…state: 1. amorphous; 2. solid crystalline; 3. gas; 4. liquid-crystal; 5. solid. 14. Viscosity of the lipid layer of membrane: 1. Corresponds to the viscosity of water; 2. Corresponds to the viscosity of vegetable oil; 3. Corresponds to the viscosity of human blood; 4. Corresponds to the viscosity of glycerol; 5. Corresponds to the viscosity of the air. 15. Modern model of the structure of membrane: 1. model of Davson and Danielli; 2. Robertson model; 3. Lili model; 4. Singer-Nicolson model; 5. Einstein model. 16. Main functions of biological membranes: 1. barrier, mechanical, matrix; 2. matrix, receptor, mechanical; 3. receptor, barrier, mechanical; 4. mechanical, receptor, electrically insulating; 5. matrix, barrier, electrically insulating. 17. Cooperative process: 1. formation of the lipid bilayer; 2. phase transition that takes place only in small area; 3. formation of water solution; 4. forming of red blood cells rouleaus; 5. potential evasion. 18. Membrane model: 1. can be presented as inductor; 2. can be presented as ohmic resistance; 3. can be presented as hydrodynamic element; 4. can be presented as plane condenser; 5. can be presented as thermodynamic element. 19. Functions of the membrane: 1. forming of a shock wave, electrical insulator; 2. transport of substances, mechanical support of a cell, electrical insulator; 3. increasing the hematocrit, forming of a shock wave; 4. mechanical support of a cell, increasing the hematocrit; 5. forming of a shock wave, mechanical support of a cell, transport of substances. 20. Tendency of lipid molecules to associate in volume structures in water solutions: 1. electrostatic force; 2. hydrophobic interaction; 3. Van der Waals force; 4. adsorption forces; 5. gravitation interaction. 21. Proteins that are on the surface of membrane: 1. peripheral; 2. integral; 3. anchor; 4. transmembrane; 5. liposomes. 22. Proteins immersed to the lipid layer: 1. peripheral; 2. integral; 3. anchor; 4. membrane; 5. liposomes. 23. Diffusing molecule without the forming of complexes with other molecules: 1. electroosmosis; 2. facilitated diffusion; 3. simple diffusion; 4. filtration; 5. osmosis. 24. Diffusing molecule with the forming of complex with a carrier: 1. electroosmosis; 2. facilitated diffusion; 3. simple diffusion; 4. filtration; 5. osmosis. 25. Composition of biological membranes: 1. DNA, fructose; 2. proteins, lipids; 3. RNA, glucose; 4. glucose, fructose; 5. ATP, DNA. 26. Transferring of water molecules through the semipermeable membrane from the area with lower concentration to the area with higher concentration of soluted substance: 1. facilitated diffusion; 2. simple diffusion; 3. simple; 4. filtration; 5. osmosis. 27. The process of substances transferring inside the cell: 1. endocytosis; 2. exocytosis; 3. phagocytosis; 4. primary-active transport; 5. secondary-active transport. 28. Transport of solid bodies inside the cell:: 1. endocytosis; 2. exocytosis; 3. phagocytosis; 4. pinocytosis. не хватает пятого варианта 29. Transport of solutions into the cell: 1. endocytosis; 2. exocytosis; 3. phagocytosis; 4. pinocytosis; 5. secondary-active transport. 30. Movable carrier of ions through the membrane: 1. valinomycin; 2. protons; 3. gramicidin; 4. electrons; 5. neutrons. 31. Immovable carrier of ions through the membrane: 1. valinomycin; 2. nigericin; 3. gramicidin; 4. electrons; 5. protons. 32. Spontaneous process of penetration from the area with lower concentration to the area with higher concentration: 1. osmosis; 2. filtration; 3. diffusion; 4. transport against the concentration gradient; 5. electroosmosis. 33. Transport of substances on the direction of concentration gradient, i.e. from the area with higher concentration to the area with lower concentration: 1. active; 2. counteracting; 3. passive; 4. potential; 6 вариантов, 2 одинаковых варианта 5. filtration; 6. active transport. 34. Types of passive transferring: 1. simple diffusion, against concentration gradient; 2. osmosis, movement against the pressure gradient; 3. osmosis, movement against the pressure gradient, filtration; 4. diffusion, osmosis, filtration, electroosmosis; 5. osmosis, movement against the temperature. 35. P=D/X 1. coefficient of membrane permeability; 2. coefficient of membrane density; 3. coefficient of membrane diffusion; 4. membrane mass concentration; 5. coefficient of membrane viscosity. 36. Transport of substances in membranes in organism that occurs with the consumption of metabolism energy: 1. passive transport of a substance; 2. active transport of a substance; 3. diffusive transport of a substance; 4. facilitated diffusive transport of a substance; 5. secondary active transport of a substance. 37. For the transferring of substances in membranes ATP energy is used. This is: 1. diffusive transport; 2. facilitated transport; 3. primary-active transport; 4. secondary active transport; 5. passive transport. 38. The ion carried by valinomycin through the membrane: 1. K+ and Na+ 2. Ca2+ 3. Cl- и OH- 4. K+ 5. Cl- 39. Nernst-Planck equation: 1. J = -D 2. J= 3. J= -D ( 4. 5. 40. Ability of ionic channels to selectively pass through the ions of one type is called: 1. selectivity; 2. conductivity; 3. transport activity; 4. diffusion; 5. filtration. 41. Main properties of ionic channels: 1. selectivity, independence of separate channels; 2. frequency dispersion, liquid viscosity; 3. dependence of channels parameters on hematocrit; 4. liquid viscosity, selectivity; 5. electrical conductivity, liquid viscosity. 42. Simple diffusion through bilipid layer obeys to the law: 1. Goldman-Hodgkin; 2. Nernst-Planck; 3. Fick; 4. Teorell; 5. Huxley. 43. Polar heads of lipids: 1. have a charge, hydrophilic, are directed outside; 2. are direct inside in the second lipid layer, don’t have a charge; 3. tend not to contact with water molecules; 4. hydrophobic, are directed inside in the second lipid layer; 5. hydrophilic, tend not to contact with water molecules. 44. Nonpolar “tails” of lipids: 1. have a charge; 2. hydrophilic; 3. hydrophobic; 4. are direct outside in the second lipid layer; 5. tend to contact with water molecules. 45. Spherical vesicles formed at the shaking of water-lipid mixture: 1. monolayer; 2. liposome; 3. bilayer lipid membrane; 4. proteoliposome; 5. single layer. 46. Transport of substances with participation of carriers differs from simple diffusion by: 1. greater solubility; 2. greater velocities of transferring; 3. lower velocities of transferring; 4. lower solubility in water; 5. lower solubility in lipids. 47. Physical quantity characterizing the ability of biological membrane to pass particular substances through itself is: 1. permeability; 2. action potential; 3. facilitated diffusion; 4. osmosis; 5. active transport. 48. Types of membrane lipids: 1. phospholipids, glycolipids, steroids; 2. carbohydrates, proteins, glycolipids; 3. amino acids, carbohydrates, steroids; 4. phospholipids, proteins; 5. neurons, amino acids. 49. Types of biological membranes: 1. neurons, cellular; 2. cellular, intracellular, basal; 3. nervous fibers, basal; 4. neurons, proteins; 5. cholesterol, proteins. 50. SI unit of flux density of diffusing substance: 1. second divided on square metre; 2. square metre divided on second; 3. metre per second; 4. mol per second; 5. mol per second divided on square metre. моль в секунду на квадратный метр 51. SI unit of the flux of diffusing substance: 1. моль в секунду на квадратный метр 2. метр в секунду 3. моль в секунду 4. квадратный метр на секунду 5. секунда на квадратный метр 52. SI unit of diffusion coefficient: 1. квадратный метр на секунду 2. секунда на квадратный метр 3. моль в секунду на квадратный метр 4. метр в секунду 5. моль в секунду 53. SI unit of permeability coefficient: 1. моль в секунду на квадратный метр 2. моль в секунду 3. метр в секунду 4. квадратный метр на секунду 5. секунда на квадратный метр 54. SI unit of concentration gradient: 1. метр в секунду 2. моль на метр в четвертой степени 3. квадратный метр на секунду 4. секунда на квадратный метр 5. метр моль в секунду 55. Direction implemented at active transport: 1. down the potential gradient; 2. down the concentration gradient; 3. against the concentration gradient; 4. down the pressure gradient; 5. against the pressure gradient. 56. Release of ions at the work of electrogenic ionic pump K-Na-АТP-ase for the complete cycle: 1. two sodium ions out of the cell; 2. three potassium ions out of the cell; 3. three sodium ions out of the cell; 4. one sodium ion out of the cell; 5. enrichment of cytoplasm with two sodium ions. 57. At the complete work cycle of electrogenic ionic pump K-Na-АТP-ase: 1. release of three potassium ions out of the cell occurs; 2. enrichment of cytoplasm with two potassium ions occurs; 3. release of one sodium ion out of the cell occurs; 4. enrichment of cytoplasm with three potassium ions occurs; 5. release of two sodium ions out of the cell occurs. 58. For the complete work cycle of electrogenic ionic pump K-Na-АТP-ase: 1. hydrolysis of five ATP molecules occurs; 2. hydrolysis of four ATP molecules occurs; 3. hydrolysis of three ATP molecules occurs; 4. hydrolysis of two ATP molecules occurs; 5. hydrolysis of one ATP molecule occurs. 59. K-Na-АТP-ase enzyme executed five complete cycles in plasmatic membrane of a red blood cell. Herewith…sodium ions were actively transported. 1. 9 2. 15 3. 6 4. 10 5. 20 60. K-Na-АТP-ase enzyme executed five complete cycles in plasmatic membrane of a red blood cell. Herewith…potassium ions were actively transported. 1. 20 2. 9 3. 10 4. 6 5. 15 61. K-Na-АТP-ase enzyme executed five complete cycles in plasmatic membrane of a red blood cell. Herewith…ATP molecules were actively hydrolysed: 1. 6 2. 10 3. 20 4. 5 5. 9 62. Medium that consists of great number of separate elements, each of them is autonomous source of energy is called: 1. active; 2. passive; 3. viscous; 4. ideal; 5. excitated. 63. Types of secondary active transport of ions: 1. transfer through the pores and facilitated diffusion; 2. simple diffusion and transfer through the pores; 3. simple diffusion, transfer through the pores and facilitated diffusion; 4. uniport, symport and antiport; 5. simple diffusion and transfer with carriers. 64. If the same charged ions of two types are transported to different sides then it is called: 1. simple diffusion; 2. transfer through the pores; 3. uniport; 4. symport; 5. antiport. 65. If unidirectional charged particles are transported towards the lower value of potential then it is called: 1. simple diffusion; 2. facilitated diffusion; 3. diffusion; 4. uniport; 5. symport. 66. Transport of oppositely charged ions in one direction is called: 1. simple diffusion; 2. facilitated diffusion; 3. transfer through the pores; 4. uniport; 5. symport. 67. Tetrodotoxin blocks the permeability of biological membrane for: 1. potassium ions; 2. sodium ions; 3. chlorine ions; 4. calcium ions; 5. water. 68. Tetraethylammonium blocks the permeability of biological membrane for: 1. potassium ions; 2. sodium ions; 3. chlorine ions; 4. calcium ions; 5. water. 69. Method of freezing with chipping includes following stages: 1. freeze, chip; 2. crystallize, heat; 3. amalgamate, crystallize; 4. chip, heat; 5. heat, crystallize. 70. Cholesterol influences to the fluidity (movability) of the membrane: 1. decreasing it; 2. decreasing it only at the increasing of temperature; 3. increasing it; 4. increasing it at the decreasing of temperature; 5. doesn’t influence. 71. For the research of dynamic properties of biological membranes spin tags and probes are widely used. Spin probe is: 1. molecule or molecular group with unpaired electrons that is added to a component of the membrane by covalent bond; 2. molecule or molecular group that is capable to fluorescence and added to a component of the membrane by non-covalent bond; 3. molecule or molecular group with unpaired electrons that is added to a component of the membrane by non-covalent bond; 4. molecule or molecular group that is capable to fluorescence and added to a component of the membrane by covalent bond; 5. molecule or molecular group containing radioactive isotopes that is added to a component of the membrane by non-covalent bond. 72. For the research of dynamic properties of biological membranes spin tags and probes are widely used. Spin tag is: 1. molecule or molecular group with unpaired electrons that is added to a component of the membrane by non-covalent bond; 2. molecule or molecular group that is capable to fluorescence and added to a component of the membrane by non-covalent bond; 3. molecule or molecular group with unpaired electrons that is added to a component of the membrane by covalent bond; 4. molecule or molecular group that is capable to fluorescence and added to a component of the membrane by covalent bond; 5. molecule or molecular group containing radioactive isotopes that is added to a component of the membrane by non-covalent bond. 73. For the research of dynamic properties of biological membranes fluorescent tags and probes are widely used. Fluorescent probe is: 1. molecule or molecular group containing radioactive isotopes that is added to a component of the membrane by non-covalent bond; 2. molecule or molecular group that is capable to fluorescence and added to a component of the membrane by covalent bond; 3. molecule or molecular group with unpaired electrons that is added to a component of the membrane by non-covalent bond; 4. molecule or molecular group with unpaired electrons that is added to a component of the membrane by covalent bond; 5. molecule or molecular group that is capable to fluorescence and added to a component of the membrane by non-covalent bond. 74. For the research of dynamic properties of biological membranes fluorescent tags and probes are widely used. Fluorescent tag is: 1. molecule or molecular group with unpaired electrons that is added to a component of the membrane by non-covalent bond; 2. molecule or molecular group that is capable to fluorescence and added to a component of the membrane by covalent bond 3. molecule or molecular group that is capable to fluorescence and added to a component of the membrane by non-covalent bond 4. molecule or molecular group containing radioactive isotopes that is added to a component of the membrane by non-covalent bond 5. molecule or molecular group with unpaired electrons that is added to a component of the membrane by covalent bond. 75. Specify the formula of substance density of uncharged particles: 1. J=-UmZFcdj/dx 2. J=P(Ci-C0) 3. J= -Ddc/dx-D/RTzFcdx/dx 4. J= -D(dc/dx+y/lc) 5. J= -C(dc/dx+y/lc) 76. Intensive thermal motion occurring on the surface of membrane bilayers: 1. passive transport; 2. simple diffusion; 3. lateral diffusion; 4. facilitated diffusion; 5. filtration. 77. Gramicidin molecule transfers across the membrane: 1. K+ и Na+ 2. Ca2+ 3. Cl- и OH- 4. Na+ 5. Cl- 78. Na+, K+ - pump transports to a cell: 1. 2Na+, and 3K+ out of the cell; 2. 2K+ and 3Na+ out of the cell; 3. 3K+, and 2Na+ out of the cell; 4. 3Na+, and 2K+ out of the cell; 5. 3Na+, and 3K+ out of the cell. 79. Diffusions equation: 1. Newton; 2. Einstein; 3. Planck; 4. Fick; 5. Goldman-Hodgkin. 80. Structural components of biomembranes: 1. proteins, lipids, carbohydrates; 2. red blood cells, white blood cells, proteins; 3. phospholipids, fats, carbohydrates. 4. haemoglobin, lipids; 5. RNA. 81. Dependence of the bilipid membrane lifetime on different factors: 1. only on membrane composition; 2. only on external conditions; 3. on membrane composition and external conditions; 4. on temperature; 5. on conformational transformations. 82. Transfer of oxygen molecules across the cell membrane: 1. simple diffusion; 2. facilitated diffusion; 3. electrodiffusion; 4. ionic transport in channels; 5. induced ionic transport. 83. Value of concentration gradient at stationary diffusion: 1. increases; 2. decreases; 3. constant; 4. equals to zero; 5. positive. 84. Nonstationary diffusion of substance concentration in any point: 1. constant; 2. equals to zero; 3. is determined by time; 4. is determined by a coordinate; 5. is determined by a coordinate and time. 85. Movable carriers of ions across the membrane provide the process of: 1. simple diffusion; 2. facilitated diffusion; 3. electrodiffusion; 4. ionic transport in channels; 5. active transport. 86. Specify the Fick’s law of diffusion: 1. 2. J=-D dc/dx 3. D=1/3 < 4. D=-1/3 < 5. F=- 87. One type of passive transport is: 1. diffusion of potassium against the concentration gradient; 2. diffusion of water from the area with high content to the area with low content; 3. symport; 4. diffusion of sodium down the potential gradient; 5. uniport. 88. Diffusion of molecules and ions in direction of their lower concentration, moving under the action of field is: 1. active transport; 2. passive transport; 3. osmosis; 4. filtration; 5. diffusion across a channel. 89. In addition to passive transport in cell membranes transfer of molecules to the area with higher concentration occurs. It is characteristics only of biological objects: 1. diffusion across a channel; 2. osmosis; 3. active transport; 4. facilitated diffusion; 5. diffusion with a carrier. 90. Membranes form subcell particles with different purpose within the cell: 1. lysosomes, axoplasm; 2. neurilemma, lysosomes; 3. mitochondria, lysosomes, ER (endoplasmic reticulum); 4. ER, carbohydrates; 5. red blood cells. 91. Membrane lipids (low molecular substances) are close by their properties to: 1. glycerols; 2. sugars; 3. fats; 4. carbohydrates; 5. alcohols. 92. Thickness of plasmatic membrane of a cell is: 1. in 5-10 times less than wavelength of green light; 2. in 50-100 times less than wavelength of green light; 3. in 5-10 times greater than wavelength of green light; 4. approximately equals to the wavelength of green light; 5. in 50-100 times greater than wavelength of green light. 93. Molecules of lipids that are in composition of biological membranes are: 1. nonpolar; 2. polar; 3. hydrophobic; 4. hydrophilic; 5. amphiphilic. 94. Viscosity of lipid part of the membrane: 1. 1 mPa·s; 2. 30-100 mPa·s; 3. 0,1 mPa·s; 4. 1 Pa·s; 5. 3 mPa. 95. Singer-Nicolson model: 1. “sandwich”; 2. unitary; 3. fluid-mosaic; 4. hydrocarbonic; 5. bilayer. 96. Transport of molecule without electrical charge across the membrane: 1. is determined only by the concentrations difference of this substance on both sides of the membrane; 2. is determined only by the sizes of substance molecules; 3. is determined only by the concentrations of other substances; 4. is determined by the concentrations difference of the substance and by the difference of electrical potentials. нет пятого варианта 97. Permeability of biological membranes for different substances: 1. ions, acids; 2. fat-soluble, water; 3. water-soluble, acids; 4. acid, water; 5. bases and acids. 98. Glycolipid: 1. provides the existence of negative electric charge on cell surfaces; 2. provides the transport of ions across the biological membranes; 3. provides the presence of channels in the membrane; 4. provides the flow of substances across the membrane; 5. provides the facilitated diffusion. 99. Proteins of the first type: 1. provides the electrostatic interaction; 2. provides phagocytosis; 3. provides the facilitated diffusion; 4. provides the presence of channels; 5. provides the Обеспечивает через биопотенциалов 100. Proteins of the second type: 1. provides the Van der Waals interaction; 2. provides the facilitated diffusion; 3. provides the presence of channels; 4. provides the existence of negative electric charge on cell surfaces;
|

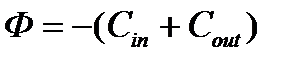 .
.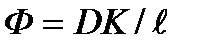 .
. .
. .
. .
.

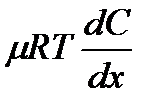
 )
)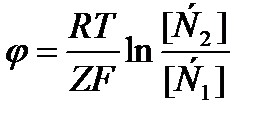

 =Dp
=Dp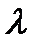 > <
> < 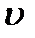 >
> >
>