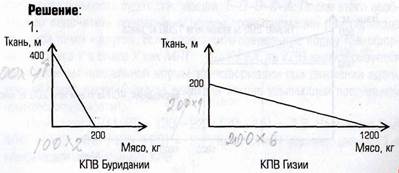
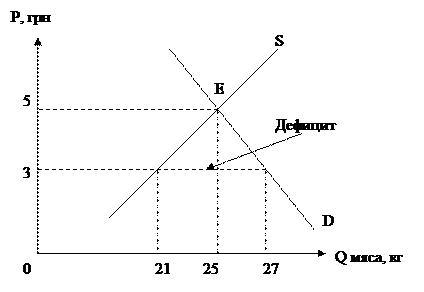
RESULTS AND DISCUSSION
A complex mixture of products results from the reaction of a methanolic solution of ammonia with artemisinin as determined by TLC. A 1H NMR spectrum of the mixture suggested the presence of a methyl ketone, but no signal characteristic of an aldehydic proton was observed. Apparently the amide nitrogen or hydroperoxide moieties form adducts with the aldehyde (e.g. 3c,d, respectively, in Scheme 1). Prolonged reaction of 2 and ammonia produces a more complex mixture containing polar products. Avery et al.4 synthesized several artemisinin derivatives by treating crude mixtures of hydroperoxides with acid. They employed Amberlyst 15 in their early work4b but in later publications reported superior yields with a mixture of aqueous sulfuric acid/silica gel (H 2SO4 /SiO2).4c Treatment of the crude reaction mixture from 2 and ammonia with H2SO4 / SiO2, as described by Avery et al.,4c produced a mixture of two products separable by column chromatography.5 The major product, 11-azaartemisinin (4), was obtained in 45% yield and a more polar product, 10-azadesoxyartemisinin (5), in 9% yield. The structural assignments of 4 and 5 were based on 1H and 13C NMR and mass spectrometric data. Additional data supporting the assigned structures were obtained employing 15NH3 in the synthesis. The 13C NMR spectra of both 15N-containing products showed that in 4 the 15N was attached to C-12 (1J = 11.9 Hz) and C-10 (1J — 10.3 Hz) and showed a long-range coupling with C-9 (3J = 5.9 Hz). In 5 the 15N was coupled to C-11 (10.4 Hz) and C-9 (11.7 Hz) and exhibited a long-range coupling to C-8 (5.9 Hz). When Amberlyst 15 was substituted for H2SCO4 / SiO2, the yield of 4 increased to 65% and compound 5 was not observed. The conversion of artemisinin into a lactam led us to attempt to prepare N-substituted 11-azaartemisinins using alkylamines instead of ammonia. The reaction of a methanolic solution of allylamine with 2 produced a mixture of products which on treatment with dilute H2SO4/SiO2 yielded N-allyl-11-azaartemisinin, 6, in 45% yield along with N-allyl-10-azadesoxyartemisinin, 7, in 15% yield. Here again, use of Amberlyst 15 produced only 6 in high yield (84%). Since 7 is an expected metabolite of 6,3a a sample of 7 will facilitate biological studies with 6 by facilitating identification of the expected metabolite. When isobutylamine was utilized in an analogous manner, N-isobutyl-11-azaartemisinin, 8, and N-isobutyl-10-azadesoxyartemisinin, 9, were obtained. Use of methylamine in methanol N-methyl-11-azaartemisinin, 10, and N-methyl-10-azadesoxyartemisinin, 11. Reaction of 2 with aromatic and heteroaromatic amines was also examined. In order to obtain N-substituted 11-azaartemisinins, it proved essential to remove any unreacted amine present in the reaction mixture prior to treatment with acid. Failure to do so resulted in formation of N-substituted 10-azadesoxy-artemisinins. Volatile amines could be removed in vacuo, whereas the less volatile amines required extraction of a methylene chloride solution of the crude reaction mixture with an aqueous citrate buffer (pH 4.5) Freshly distilled benzylamine and 2 with the modified workup yielded 13. Reaction of 2 with the heterocyclic amines, 2-(aminomethyl)pyridine, 2-(aminomethyl)-thiophene, and 2-(aminomethyl)furan, followed by acid treatment yielded compounds 14 - 16. In exploring the use of 11-azaartemisinins as potential intermediates for the preparation of antimalarial drugs, several reactions of 4 and 6 were investigated. Reaction of compound 4 with allyl bromide in the presence of silver oxide yielded a compound isomeric with 6. Its structure has been assigned as that of the O-allyl derivative 12 based on an analysis of its 1H and 13C NMR spectra, mass spectrometric data, and the known reactivity of amides with alkyl halides in the presence of base.6 Ozonolysis of the double bond in 6 yielded aldehyde 17. The above N-substituted 11-azaartemisinins were screened against a chloroquine-resistant strain (FCR3) of P. falciparum using a previously published8 modification of the method of Desjardins et al. 9 The in vitro test results are summarized in Table 1 and demonstrate that replacement of the lactone moiety of 2 by a lactam, as in 4, yields an antimalarial drug with equivalent biological activity. The antimalarial activities of 8, 14, and 17 were 1 order of magnitude greater than that of artemisinin. These findings are consistent with those found by Avery et al.4a for their desmethylartemisinin derivatives, i.e., the antimalarial activities of lactams were as great as or greater than that of artemisinin. The presence of the endoperoxide for antimalarial activity was essential as with other artemisinin derivatives; 3a the oxides possess no significant anti-malarial activity. Although additional data are needed for a precise comparison of the relative in vitro activities of 17 and 2, we proceeded to prepare sufficient quantities of 17 for in vivo testing. The in vivo test data are given in Table 2, which shows that 17 is at least 4 times more active than 2; i.e., it is approximately as active as β-arteether. Neither recent clinical trials2a,b,d,e nor earlier clinical studies in China2c found artemisinin derivatives to be toxic. However, reports by Brewer et al.7 indicate that repeated administration of β-arteether, 1b, produces neurotoxic reactions in animals. In evaluating the potential of 17 as an antimalarial drug, data on its toxicity and bioavailability will be required. Additional azaartemisinin derivatives will be prepared as part of our planned structure – activity relationship (SAR) studies.
Sample 4
|